Question: For example, if the normal boiling point (i.e., boiling point at 760 torr, P1), for a liquid is known (T1), the Clausius-Clapeyron equation can

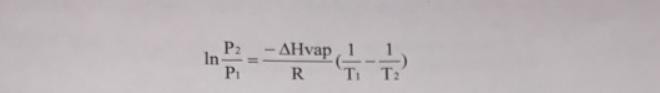
For example, if the normal boiling point (i.e., boiling point at 760 torr, P1), for a liquid is known (T1), the Clausius-Clapeyron equation can be used to calculate the boiling point (T2) at an external pressure (P2) different from 760 torr. Note: in the Clausius-Clapeyron equation, pressure should be expressed in torr, temperature in degrees Kelvin, heat of vaporization in Joules, and R = 8,314 J/mol K. If the heat of a. The normal boiling point for water is 100 C. vaporization (AHvap) for water is 40.7 kJ, calculate the boiling point of water (in C) at 25 torr. b. At 25 C and 25 torr, does water boil? Explain your reasoning. c. What assumption must be made when the Clausius-Clapeyron equation is used to calculate the boiling point of a liquid at a pressure different from 760 torr? d. Because the heat of vaporization is not the same for all substances, a pressure-temperature nomograph has one "built in" (i.e., the heat of vaporization is "forced" to be the same for all substances). Nevertheless, the nomograph can be quite useful for estimating boiling points at reduced pressure. For the vacuum distillation of the pyrolysate in Part II of the experiment, a water aspirator is used to reduce the pressure inside the distillation apparatus. If the water aspirator reduces the pressure inside the apparatus to 25 torr, and the pyrolysate has a boiling point of 70 C at this pressure, what is the approximate boiling point of the pyrolysate at atmospheric pressure (i.e., 760 torr)? Note: Use the pressure- temperature nomograph in Mayo et al, p. 49. e. Based on the approximate boiling point you determined in part d, could the pyrolysis be carried out at atmospheric pressure (i.e., as a simple distillation)? In other words, is a vacuum distillation necessary? Explain your reasoning. In P2 -AHvap P R
Step by Step Solution
3.42 Rating (158 Votes )
There are 3 Steps involved in it
a The ClausiusClapeyron equation is given by lnP2P1 HvapR 1T2 1T1 where P1 760 torr normal boiling point T1 100 K normal boiling point Hvap 407 kJmol R 8314 JmolK P2 25 torr external pressure We need ... View full answer

Get step-by-step solutions from verified subject matter experts


