Question: 2-)Elementary reversible liquid phase reaction is carried out in continuous flow reactors. Inert I and A are fed to the reactor. Volumetric flow rate is
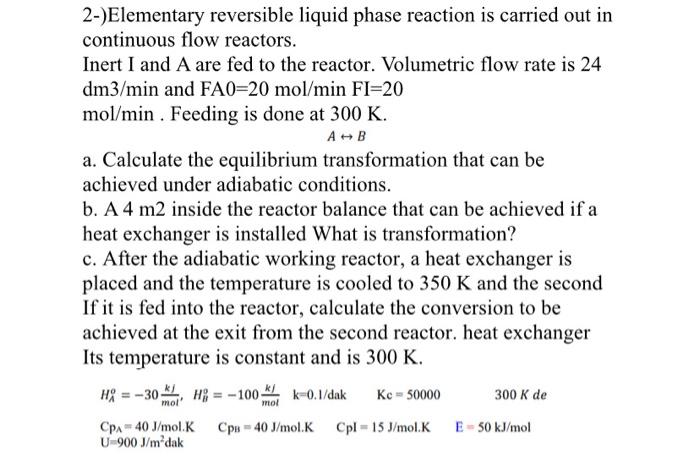
2-)Elementary reversible liquid phase reaction is carried out in continuous flow reactors. Inert I and A are fed to the reactor. Volumetric flow rate is 24 dm3/min and FA0=20mol/minFI=20 mol/min. Feeding is done at 300K. AB a. Calculate the equilibrium transformation that can be achieved under adiabatic conditions. b. A 4m2 inside the reactor balance that can be achieved if a heat exchanger is installed What is transformation? c. After the adiabatic working reactor, a heat exchanger is placed and the temperature is cooled to 350K and the second If it is fed into the reactor, calculate the conversion to be achieved at the exit from the second reactor. heat exchanger Its temperature is constant and is 300K. HAo=30molkj,HBo=100molkjk=0.l/dakKc=50000300KdeCpAp=40J/mol.KCpB=40J/mol.KCpl=15J/mol.KE=50kJ/molU=900J/m2dak
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


