Question: 2s electron feels Zeff 1 1- 1- Li 3+ shielding 1- Following the example of Li above, fill in the following table for group 1
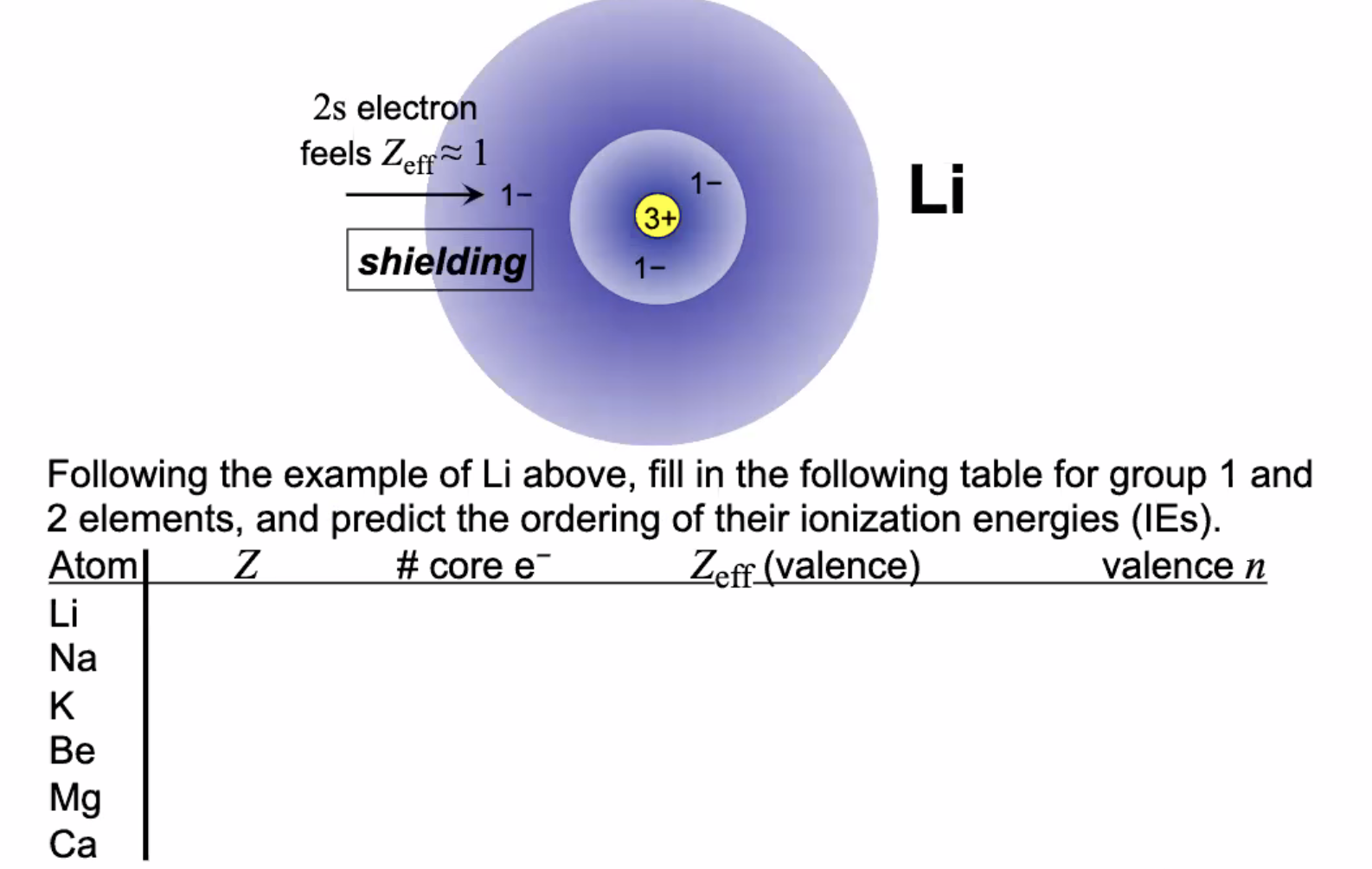
2s electron feels Zeff 1 1- 1- Li 3+ shielding 1- Following the example of Li above, fill in the following table for group 1 and 2 elements, and predict the ordering of their ionization energies (IES). Atoml Z #core e- Zeff (valence) valence n Li Na K Be Mg Ca
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


