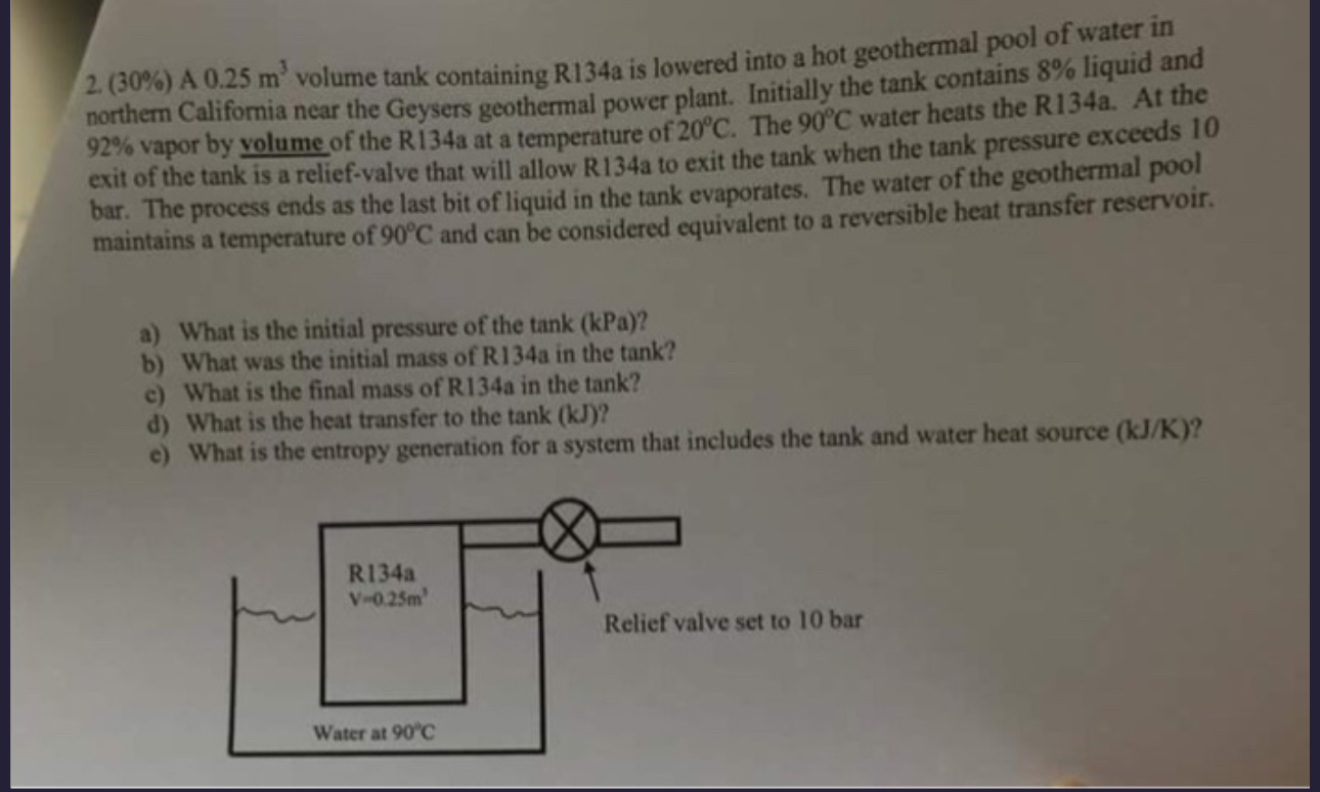
Question: ( 3 0 % ) A 0 . 2 5 m 3 volume tank containing R 1 3 4 a is lowered into a hot
A volume tank containing Ra is lowered into a hot geothermal pool of water in northem Califormia near the Geysers geothermal power plant. Initially the tank contains liquid and vapor by volume of the Ra at a temperature of The water heats the Ra At the exit of the tank is a reliefvalve that will allow Ra to exit the tank when the tank pressure exceeds bar. The process ends as the last bit of liquid in the tank evaporates. The water of the geothermal pool maintains a temperature of and can be considered equivalent to a reversible heat transfer reservoir.
a What is the initial pressure of the tank kPa
b What was the initial mass of Ra in the tank?
c What is the final mass of Ra in the tank?
d What is the heat transfer to the tank kJ
e What is the entropy generation for a system that includes the tank and water heat source
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


