Question: ( 3 0 points ) Quantum defects in alkali atoms The effective quantum number n * * is equal to n - n l ,
points Quantum defects in alkali atoms The effective quantum number is equal to
where is the quantum defect. Using the numerical method you implemented in
Problem you will now calculate the quantum defects in sodium.
a points The potential of an alkali atom can be approximated by the Coulomb potential
with central charge for tilde where the mean radius the highest closed
electron shell, and the Coulomb potential with charge for offset constant
added the potential inside ensure continuity the potential. Justify why the
aforementioned approximation valid for alkali atoms.
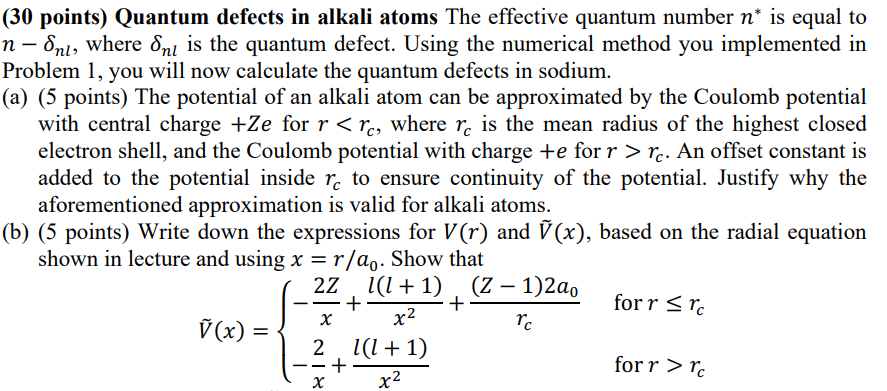
points Write down the expressions for and tilde based the radial equation
shown lecture and using Show that
tilde
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


