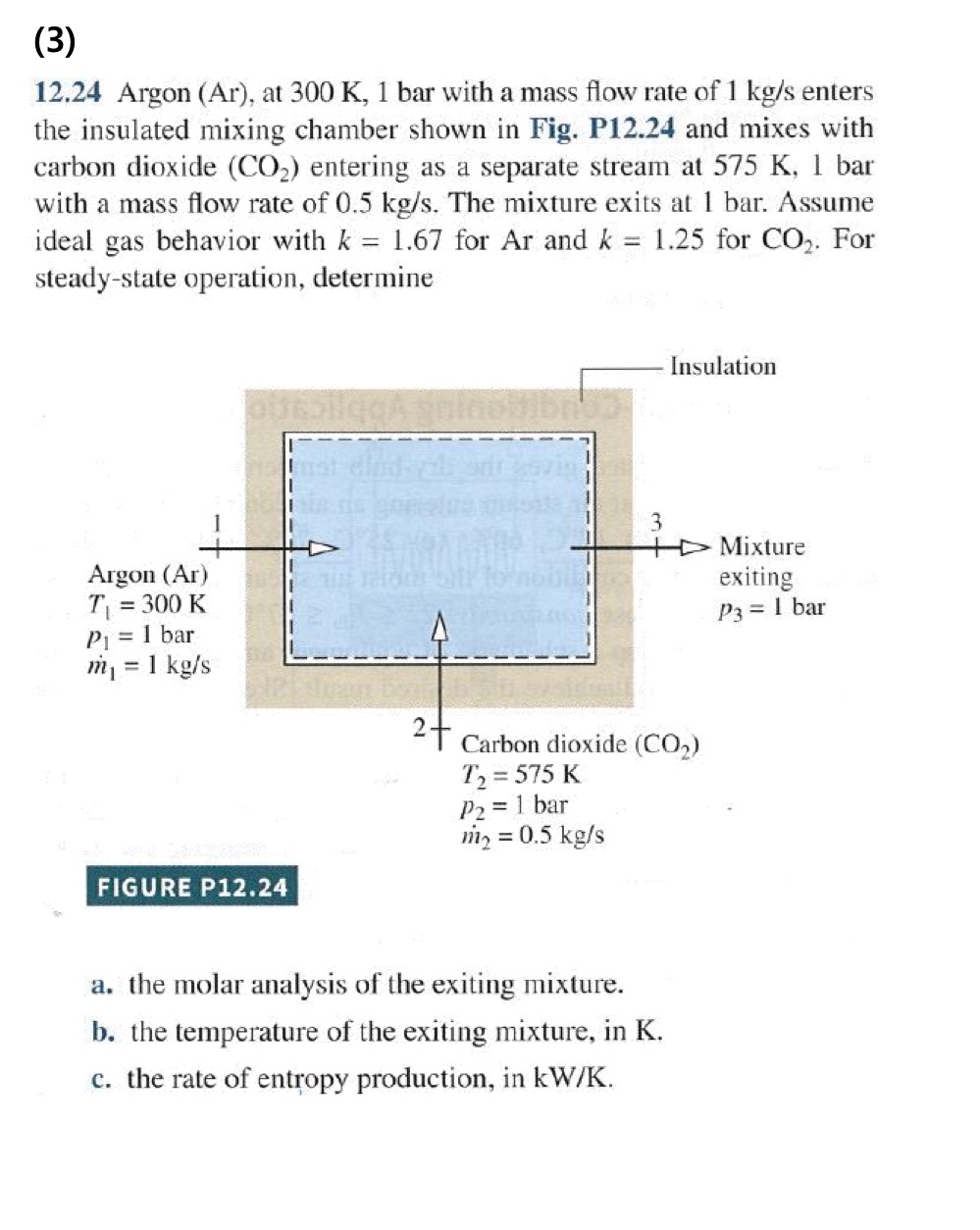
Question: ( 3 ) 1 2 . 2 4 Argon ( Ar ) , at 3 0 0 K , 1 bar with a mass flow
Argon Ar at bar with a mass flow rate of enters the insulated mixing chamber shown in Fig. P and mixes with carbon dioxide entering as a separate stream at bar with a mass flow rate of The mixture exits at bar. Assume ideal gas behavior with for Ar and for For steadystate operation, determine
a the molar analysis of the exiting mixture.
b the temperature of the exiting mixture, in K
c the rate of entropy production, in
Argon Ar at bar with a mass flow rate of enters the insulated mixing chamber shown in Fig. P and mixes with carbon dioxide entering as a separate stream at bar with a mass flow rate of The mixture exits at bar. Assume ideal gas behavior with for Ar and for For steadystate operation, determine
a the molar analysis of the exiting mixture.
b the temperature of the exiting mixture, in K
c the rate of entropy production, in
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


