Question: 3. (10 points) A scientist is trying to determine if an unknown substance is calcium carbonate (CaCO3) or calcium sulfate (CaSO4) or something else entirely.
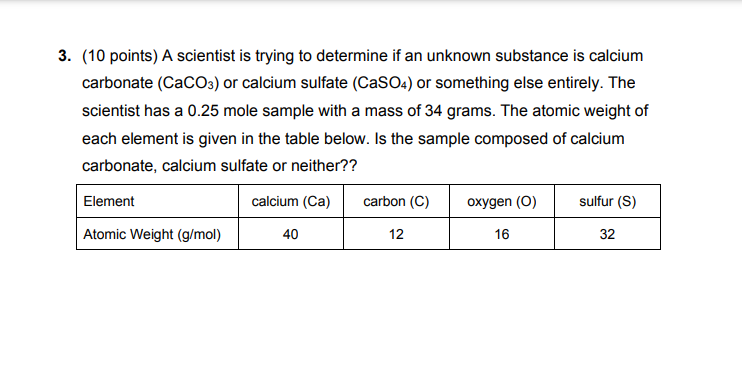
3. (10 points) A scientist is trying to determine if an unknown substance is calcium carbonate (CaCO3) or calcium sulfate (CaSO4) or something else entirely. The scientist has a 0.25 mole sample with a mass of 34 grams. The atomic weight of each element is given in the table below. Is the sample composed of calcium carbonate, calcium sulfate or neither?? Element calcium (Ca) carbon (C) oxygen (0) sulfur (S) Atomic Weight (g/mol) 40 12 16 32
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


