Question: 3) (10 pts) a. Draw the expanded Lewis structure for the following condensed formula (CH3)3CCH2CO2CH2CH(OH)CH2CH3 3b. Draw the bondline corresponding to this molecule 4. (10
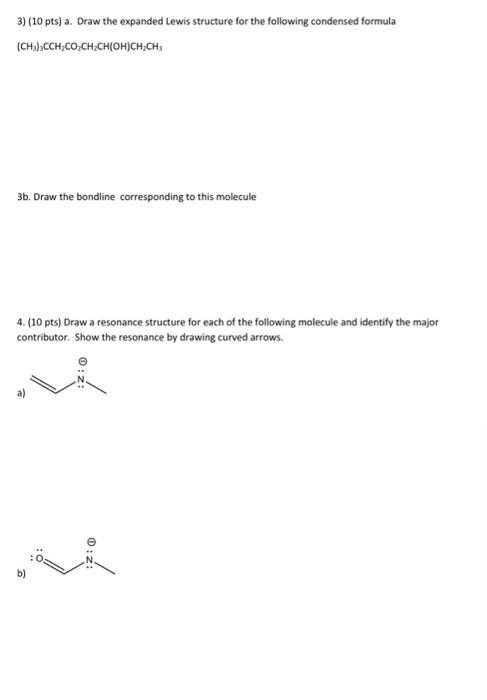
3) (10 pts) a. Draw the expanded Lewis structure for the following condensed formula (CH3)3CCH2CO2CH2CH(OH)CH2CH3 3b. Draw the bondline corresponding to this molecule 4. (10 pts) Draw a resonance structure for each of the following molecule and identify the major contributor. Show the resonance by drawing curved arrows. a) b)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


