Question: 3. (20 Point) The elementary gas reaction 3A + B + 2C is carried out isothermally in a PFR with no pressure drop. The feed
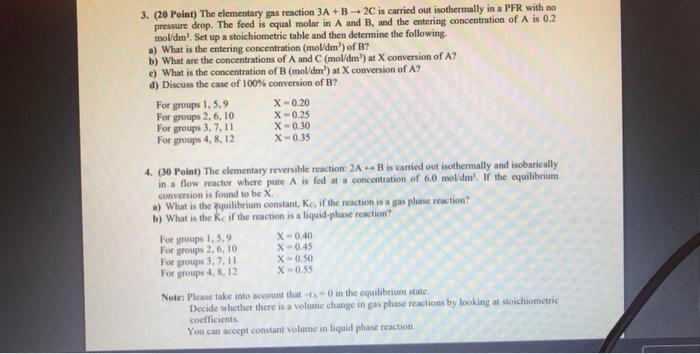
3. (20 Point) The elementary gas reaction 3A + B + 2C is carried out isothermally in a PFR with no pressure drop. The feed is equal molar in A and B, and the entering concentration of A is 0.2 mol/dm'. Set up a stoichiometric table and then determine the following a) What is the entering concentration (mol/dm) of B? b) What are the concentrations of A and C (mol/dm) at X conversion of A? c) What is the concentration of B (mol/dm') at X conversion of A? d) Discuss the case of 100% conversion of B? For groups 1.5.9 X-0.20 For groups 2, 6, 10 X-0.25 For groups 3, 7.11 X-0.30 For groups 4,8,12 X-0.35 4. (30 Point) The clementary reversible reaction: 2A - B is carried out isothermally and isobarically in a flow reactor where pure A is fed at a concentration of 6.0 mol/dml. If the equilibrium conversion is found to be X a) What is the quilibrium constant, Ke, if the reaction is a gasphase reaction? b) What is the ke if the reaction is a liquid-phase reaction? For groups 1.5.9 X -0.40 For groups 2.6,10 X-0.45 For groups 3, 7, 11 X-0.50 For groups 4,8,12 X -055 Note: Please take into account that in the equilibrium state Decide whether there is a volume change in gasphase reactions by looking at stoichiometric coefficients You can accept constant volume in liquid phase reaction
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


