Question: 3. (25 points) The figure below shows 4 special processes for a 1mol ideal monatomic gas. Fill in the blanks in the table. The initial
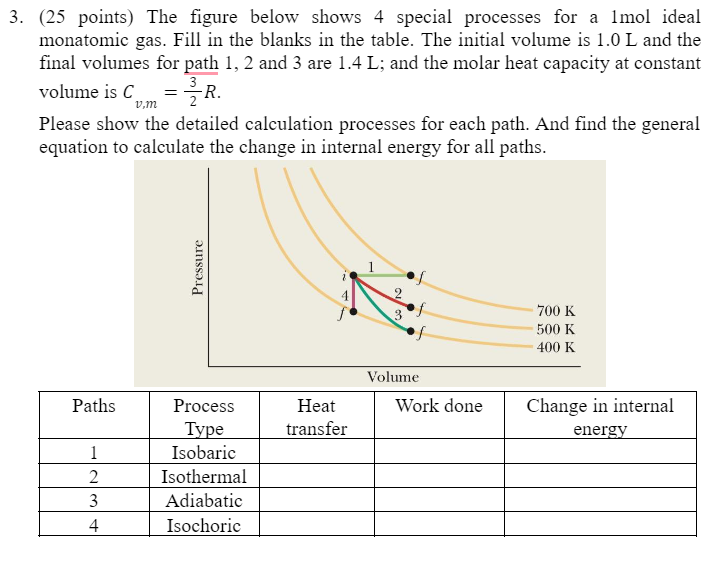
3. (25 points) The figure below shows 4 special processes for a 1mol ideal monatomic gas. Fill in the blanks in the table. The initial volume is 1.0L and the final volumes for path 1,2 and 3 are 1.4L; and the molar heat capacity at constant volume is Cv,m=23R. Please show the detailed calculation processes for each path. And find the general equation to calculate the change in internal energy for all paths. 3. (25 points) The figure below shows 4 special processes for a 1mol ideal monatomic gas. Fill in the blanks in the table. The initial volume is 1.0L and the final volumes for path 1,2 and 3 are 1.4L; and the molar heat capacity at constant volume is Cv,m=23R. Please show the detailed calculation processes for each path. And find the general equation to calculate the change in internal energy for all paths
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


