Question: 3. ( 30 points) The following data were obtained for a second order reversible constant density reaction, A+BR+S, with CA0=CB0=201gmole and CR0=CS0=0. a. Explain why
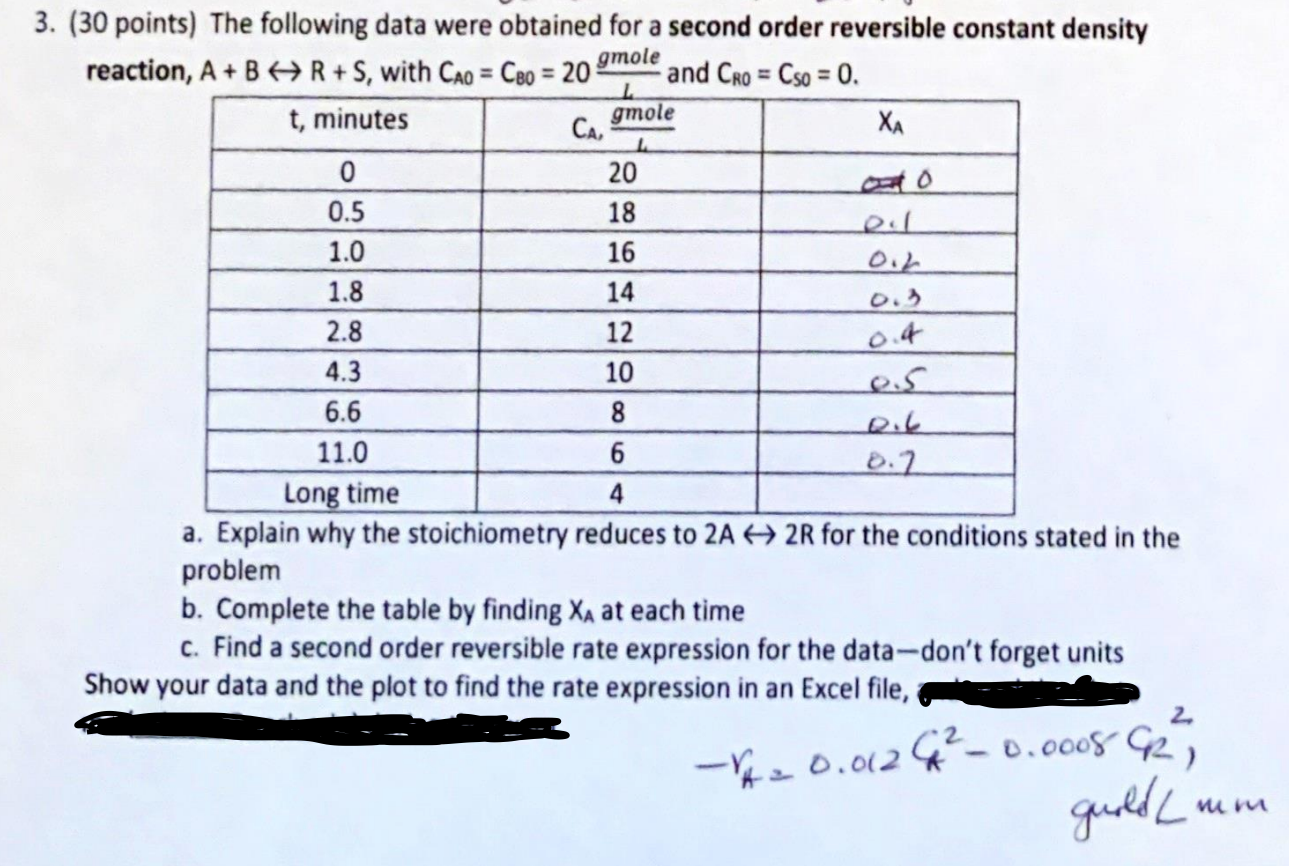
3. ( 30 points) The following data were obtained for a second order reversible constant density reaction, A+BR+S, with CA0=CB0=201gmole and CR0=CS0=0. a. Explain why the stoichiometry reduces to 2A2R for the conditions stated in the problem b. Complete the table by finding XA at each time c. Find a second order reversible rate expression for the data-don't forget units Show your data and the plot to find the rate expression in an Excel file
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


