Question: 3. (35 points) In the following process for condensing methanol vapor from air, most of the entering methanol is liquefied in this steady state process,
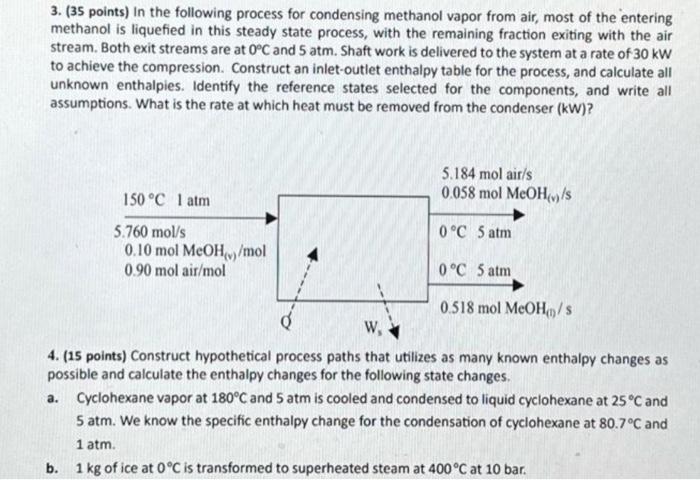
3. (35 points) In the following process for condensing methanol vapor from air, most of the entering methanol is liquefied in this steady state process, with the remaining fraction exiting with the air stream. Both exit streams are at 0C and 5atm. Shaft work is delivered to the system at a rate of 30kW to achieve the compression. Construct an inlet-outlet enthalpy table for the process, and calculate all unknown enthalpies. Identify the reference states selected for the components, and write all assumptions. What is the rate at which heat must be removed from the condenser (kW)? 4. (15 points) Construct hypothetical process paths that utilizes as many known enthalpy changes as possible and calculate the enthalpy changes for the following state changes. a. Cyclohexane vapor at 180C and 5 atm is cooled and condensed to liquid cyclohexane at 25C and 5atm. We know the specific enthalpy change for the condensation of cyclohexane at 80.7C and 1atm. b. 1kg of ice at 0C is transformed to superheated steam at 400C at 10 bar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


