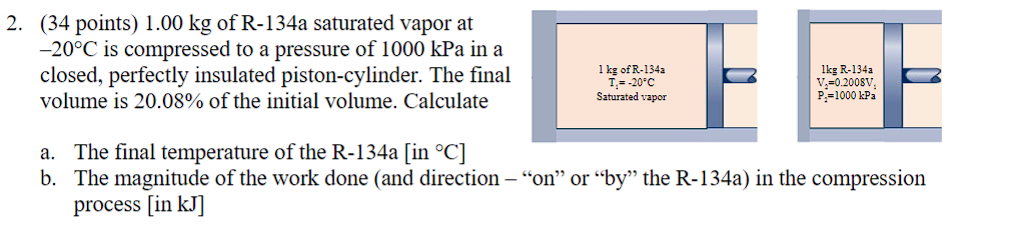
Question: ( 3 4 points ) 1 . 0 0 kg of R - 1 3 4 a saturated vapor at - 2 0 C is
points kg of a saturated vapor at
is compressed to a pressure of kPa in a
closed, perfectly insulated pistoncylinder. The final
volume is of the initial volume. Calculate
a The final temperature of the Ra in :
b The magnitude of the work done and direction on or by the Ra in the compression
process in kJ
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


