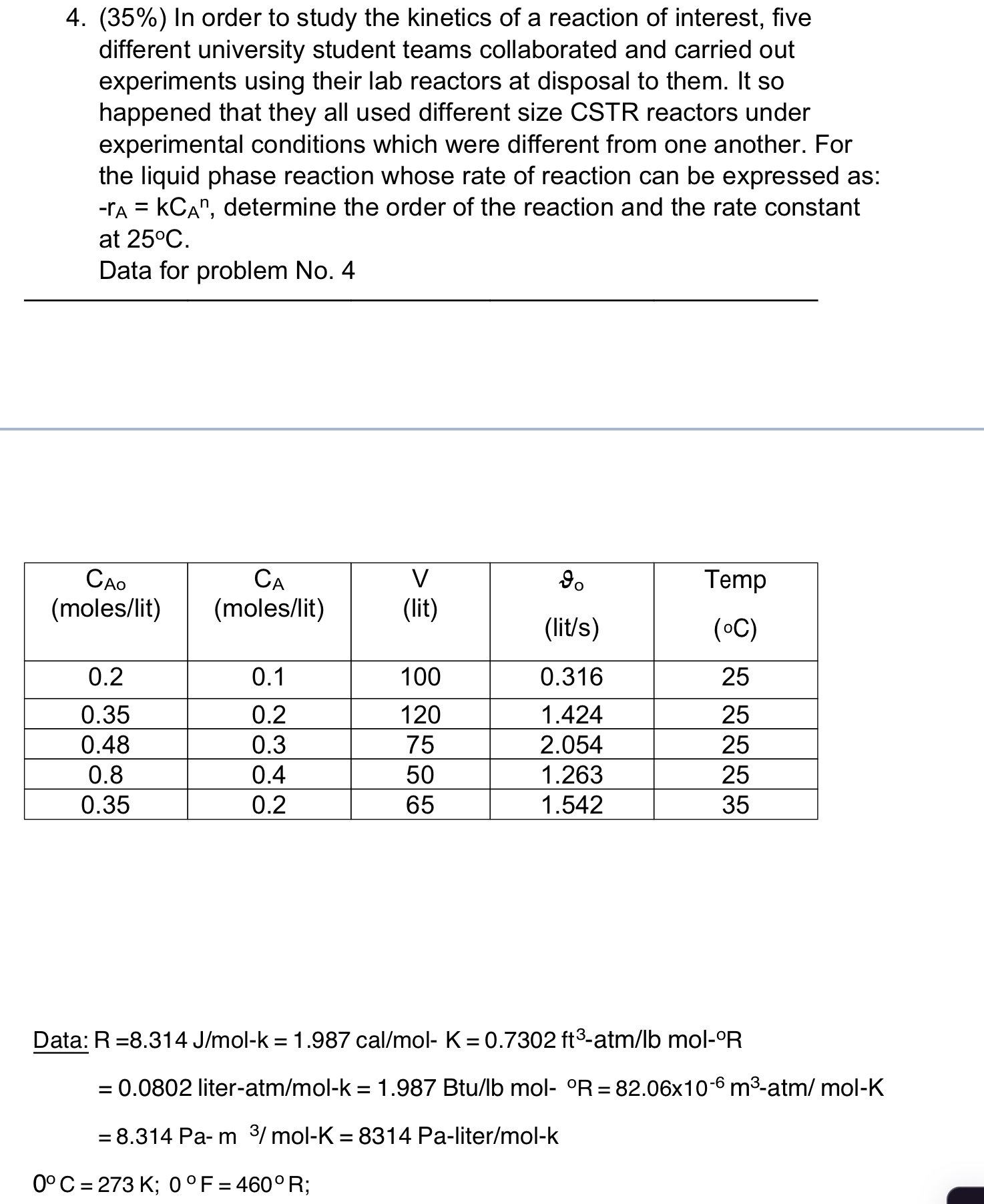
Question: ( 3 5 % ) In order to study the kinetics of a reaction of interest, five different university student teams collaborated and carried out
In order to study the kinetics of a reaction of interest, five different university student teams collaborated and carried out experiments using their lab reactors at disposal to them. It so happened that they all used different size CSTR reactors under experimental conditions which were different from one another. For the liquid phase reaction whose rate of reaction can be expressed as: determine the order of the reaction and the rate constant at
Data for problem No
tabletable
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


