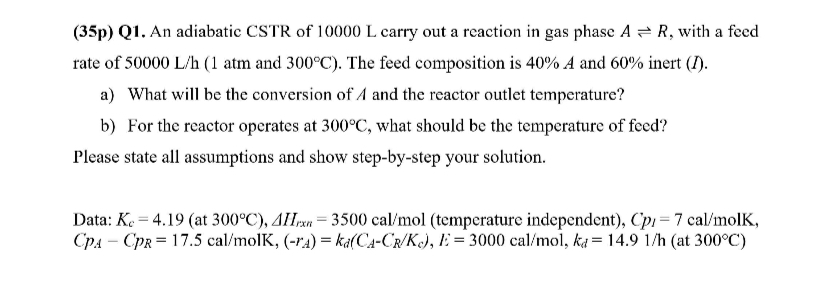
Question: ( 3 5 p ) Q 1 . An adiabatic CSTR of 1 0 0 0 0 L carry out a reaction in gas phase
p Q An adiabatic CSTR of carry out a reaction in gas phase with a feed rate of atm and The feed composition is A and inert I
a What will be the conversion of A and the reactor outlet temperature?
b For the reactor operates at what should be the temperature of feed?
Please state all assumptions and show stepbystep your solution.
Data: at temperature independent Cpp olK, at :
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


