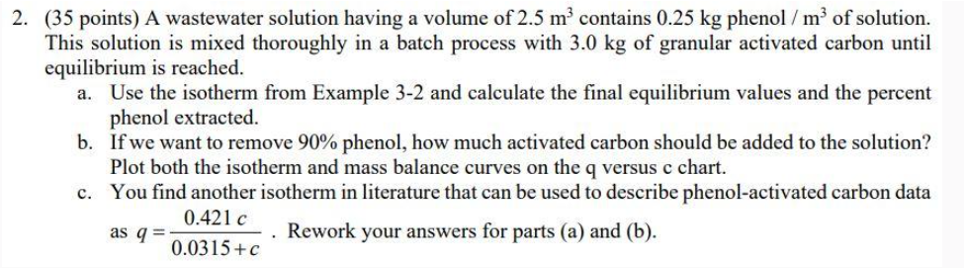
Question: ( 3 5 points ) A wastewater solution having a volume of 2 . 5 m 3 contains 0 . 2 5 k g phenol
points A wastewater solution having a volume of contains phenol of solution.
This solution is mixed thoroughly in a batch process with of granular activated carbon until
equilibrium is reached.
a Use the isotherm from Example and calculate the final equilibrium values and the percent
phenol extracted.
b If we want to remove phenol, how much activated carbon should be added to the solution?
Plot both the isotherm and mass balance curves on the q versus chart.
c You find another isotherm in literature that can be used to describe phenolactivated carbon data
as Rework your answers for parts a and b
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


