Question: 3. (5 points) The initial reaction rate for A+BC+D was measured as a function of temperature when the concentrations of A and B were 2

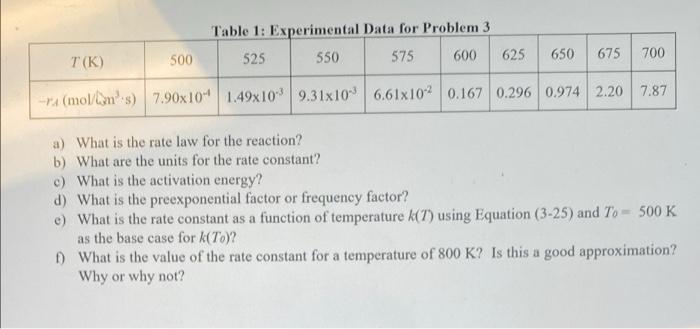
3. (5 points) The initial reaction rate for A+BC+D was measured as a function of temperature when the concentrations of A and B were 2 M and 1 M, respectively. Assume that the reaction is first order in A and order in B. The data are in Table 1. Use Excel as to analyze the data and to plot Indk) vs. 1/7. Answer the following questions by showing your work on paper Table 1: Experimental Data for Problem 3 525 550 575 600 625 500 650 675 700 T(K) --(mol/lin's) 7.90x10" 1.49x109,31x10 6.61x102 0.167 0.296 0.974 2.20 7.87 a) What is the rate law for the reaction? b) What are the units for the rate constant? c) What is the activation energy? d) What is the preexponential factor or frequency factor? e) What is the rate constant as a function of temperature k(7) using Equation (3-25) and To - 500 K as the base case for k(To)? 1) What is the value of the rate constant for a temperature of 800 K? Is this a good approximation? Why or why not
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


