Question: 3 . 6 . Our next research experiment is to be carried out in a vertical, cylindrical reactor 0 . 0 9 2 9 m
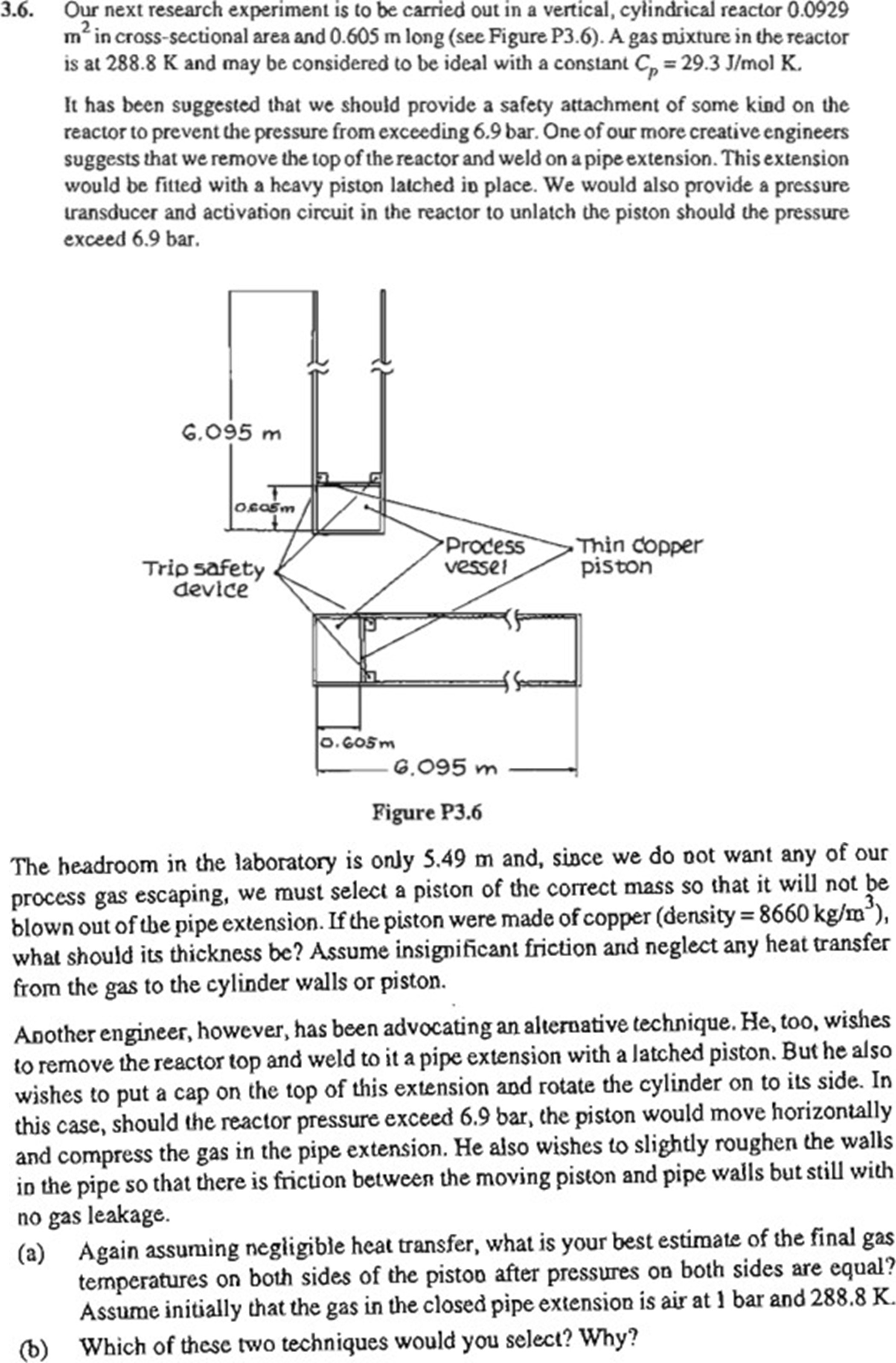
Our next research experiment is to be carried out in a vertical, cylindrical reactor in crosssectional area and long see Figure P A gas mixture in the reactor is at and may be considered to be ideal with a constant olK.
It has been suggested that we should provide a safety attachment of some kind on the reactor to prevent the pressure from exceeding One of our more creative engineers suggests that we remove the top of the reactor and weld on a pipe extension. This extension would be fitted with a heavy piston latched in place. We would also provide a pressure transducer and activation circuit in the reactor to unlatch the piston should the pressure exceed bar.
The headroom in the laboratory is only and, since we do not want any of our process gas escaping, we must select a piston of the correct mass so that it will not be blown out of the pipe extension. If the piston were made of copper density what should its thickness be Assume insignificant friction and neglect any heat transfer from the gas to the cylinder walls or piston.
Another engineer, however, has been advocating an alternative technique. He too, wishes to remove the reactor top and weld to it a pipe extension with a latched piston. But he also wishes to put a cap on the top of this extension and rotate the cylinder on to its side. In this case, should the reactor pressure exceed the piston would move horizontally and compress the gas in the pipe extension. He also wishes to slightly roughen the walls in the pipe so that there is friction between the moving piston and pipe walls but still with no gas leakage.
a Again assuming negligible heat transfer, what is your best estimate of the final gas temperatures on both sides of the piston after pressures on both sides are equal? Assume initially that the gas in the closed pipe extension is air at and
b Which of these two techniques would you select? Why?
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


