Question: 3. (6 pts) Using the periodic table, indicate which element would have each of the following properties. For each question, there may be more than
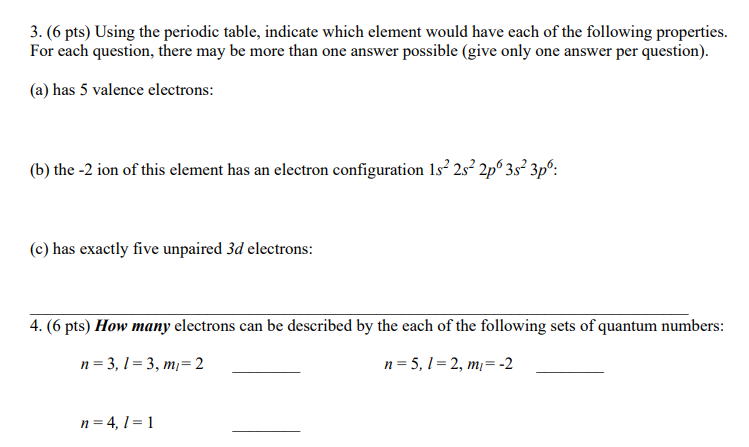
3. (6 pts) Using the periodic table, indicate which element would have each of the following properties. For each question, there may be more than one answer possible (give only one answer per question). (a) has 5 valence electrons: (b) the -2 ion of this element has an electron configuration 1s 257 2p 357 3p: (c) has exactly five unpaired 34 electrons: 4. (6 pts) How many clectrons can be described by the each of the following sets of quantum numbers: n=31=3 m=2 n=S51=2 m=-2 =4 =1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


