Question: 3. (a) Define a reducing agent in terms of electrons. [1] (b) In the following representation of a cell, label each electrode with a +
![3. (a) Define a reducing agent in terms of electrons. [1]](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f90df794a50_07166f90df73641b.jpg)

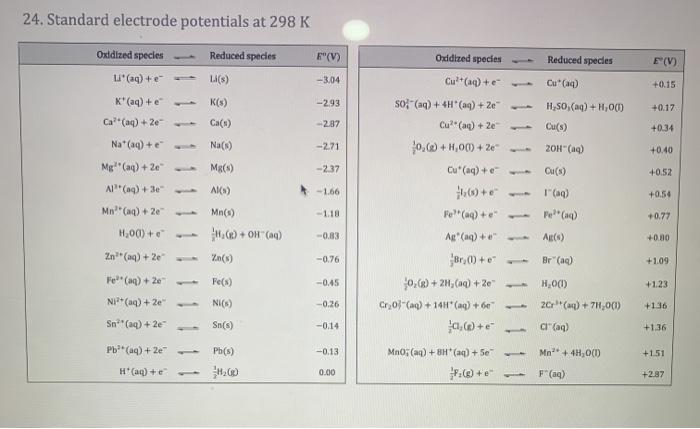
3. (a) Define a reducing agent in terms of electrons. [1] (b) In the following representation of a cell, label each electrode with a + or a - sign, as appropriate, and draw an arrow on the connecting wire to indicate the direction of electron flow. (Refer to Table 15 of the Chemistry Data Booklet.) [2] Voltmeter Ni(s)-> +Al(s) Ni(aq) Al" (aq) (c) (i) Write the balanced equation for the spontaneous reaction in the above cell. [2] (11) Calculate the standard cell potential. (2) 15. Spectrochemical series Ligands can be arranged in a spectrochemical series according to the energy difference they produce between the two sets of d-orbitals in an octahedral complex. 1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


