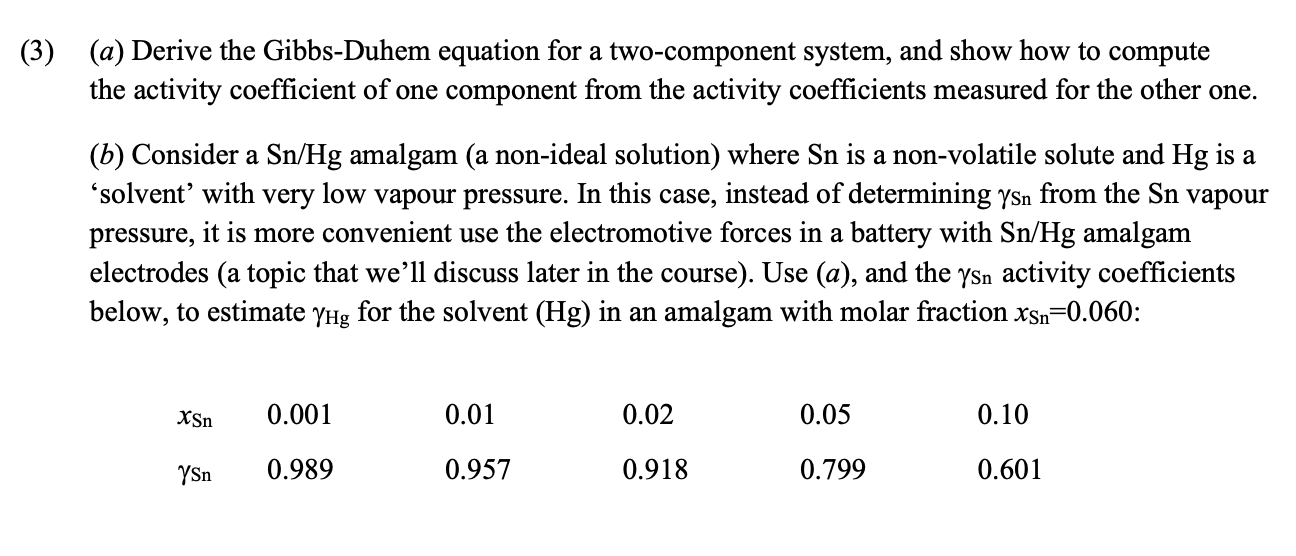
Question: ( 3 ) ( a ) Derive the Gibbs - Duhem equation for a two - component system, and show how to compute the activity
a Derive the GibbsDuhem equation for a twocomponent system, and show how to compute
the activity coefficient of one component from the activity coefficients measured for the other one.
b Consider a amalgam a nonideal solution where is a nonvolatile solute and is a
'solvent' with very low vapour pressure. In this case, instead of determining from the vapour
pressure, it is more convenient use the electromotive forces in a battery with amalgam
electrodes a topic that we'll discuss later in the course Use a and the activity coefficients
below, to estimate for the solvent in an amalgam with molar fraction :
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


