Question: 3. After data collection, a scientist plotted the graphs below to determine the rate law for the following reaction NO2(g)>NO(g)+1/2O2(g) What is the rate law
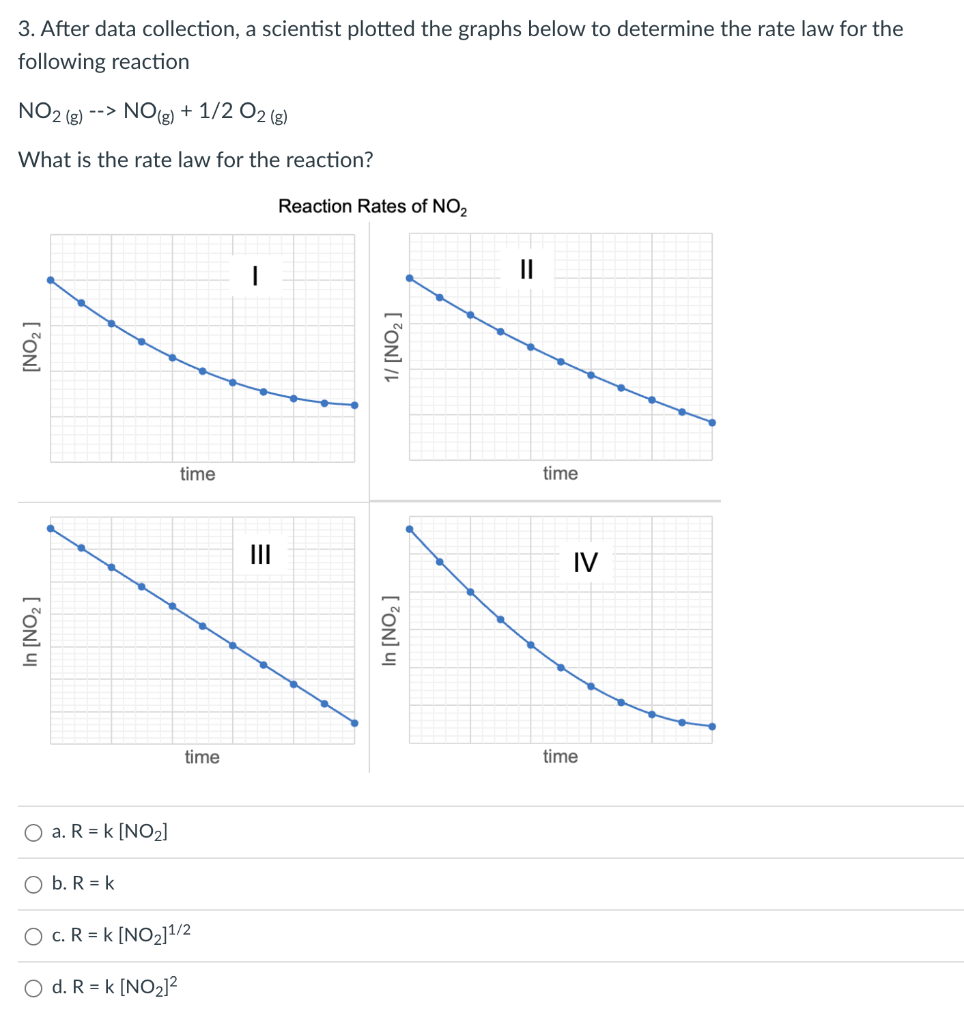
3. After data collection, a scientist plotted the graphs below to determine the rate law for the following reaction NO2(g)>NO(g)+1/2O2(g) What is the rate law for the reaction? Reaction Rates of NO2 a. R=k[NO2] b. R=k c. R=k[NO2]1/2 d. R=k[NO2]2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


