Question: 3. Assume that a structure crystallizes in the 2-D structure shown below. a) Including only nearest neighbor effects, compute the equilibrium separation of the
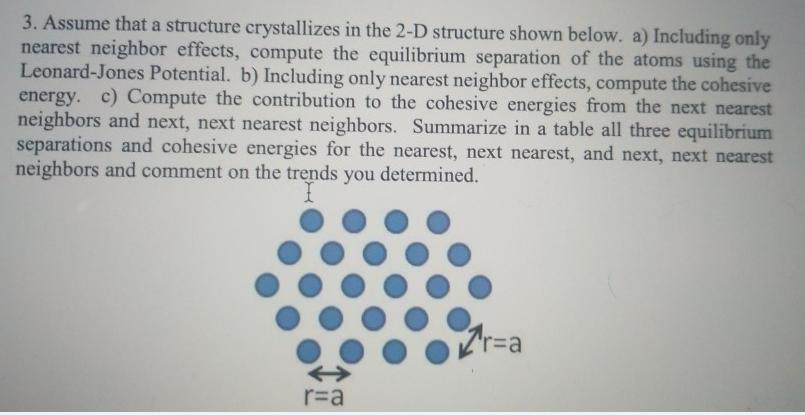
3. Assume that a structure crystallizes in the 2-D structure shown below. a) Including only nearest neighbor effects, compute the equilibrium separation of the atoms using the Leonard-Jones Potential. b) Including only nearest neighbor effects, compute the cohesive energy. c) Compute the contribution to the cohesive energies from the next nearest neighbors and next, next nearest neighbors. Summarize in a table all three equilibrium separations and cohesive energies for the nearest, next nearest, and next, next nearest neighbors and comment on the trends you determined. "r%3Da r=a 3. Assume that a structure crystallizes in the 2-D structure shown below. a) Including only nearest neighbor effects, compute the equilibrium separation of the atoms using the Leonard-Jones Potential. b) Including only nearest neighbor effects, compute the cohesive energy. c) Compute the contribution to the cohesive energies from the next nearest neighbors and next, next nearest neighbors. Summarize in a table all three equilibrium separations and cohesive energies for the nearest, next nearest, and next, next nearest neighbors and comment on the trends you determined. "r%3Da r=a
Step by Step Solution
3.30 Rating (150 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


