Question: 3 Biologics Case Study #3: Transfer to a CMO for Evaluation at Laboratory Scale, Prior to Transfer to Pilot and GMP Scale An mAb purification
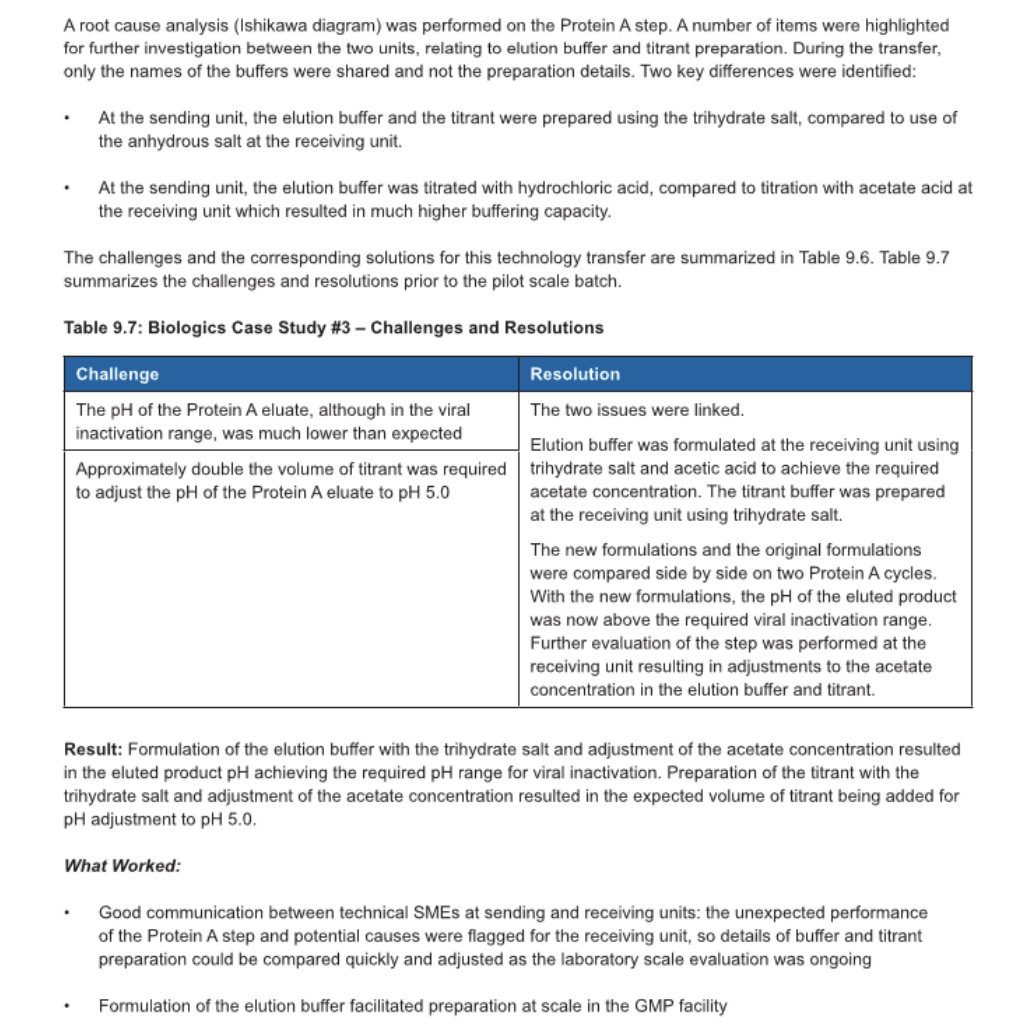
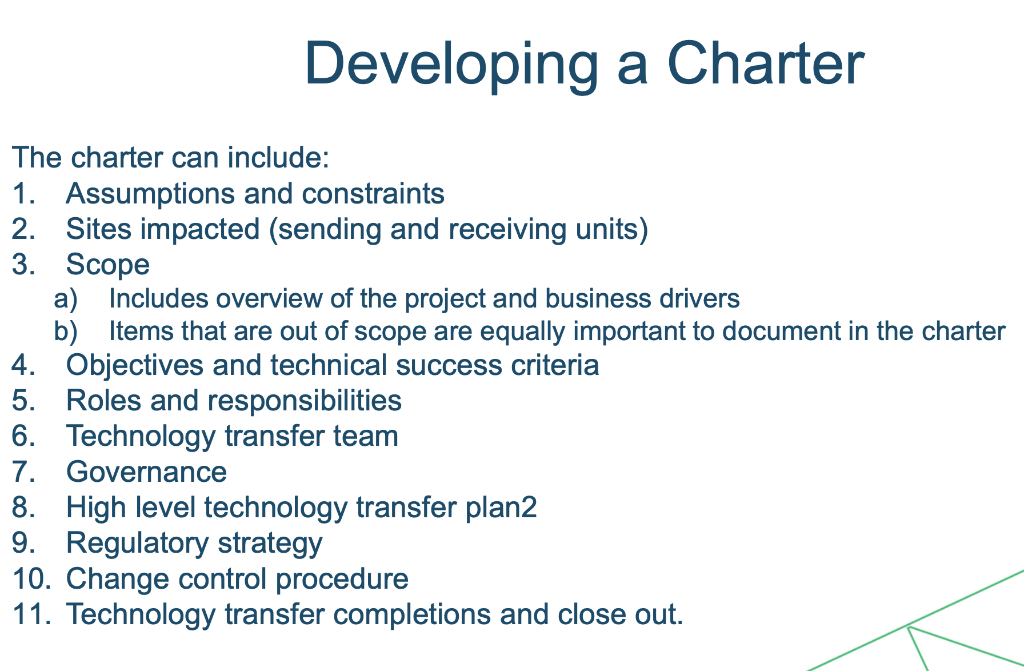
3 Biologics Case Study \#3: Transfer to a CMO for Evaluation at Laboratory Scale, Prior to Transfer to Pilot and GMP Scale An mAb purification process was developed at a small biotechnology company and transferred to a CMO for evaluation at laboratory scale, prior to scale-up to a non-GMP pilot and first GMP clinical batch. The process consisted of a Protein A chromatography step, low pH virus inactivation, and pH adjustment followed by cation exchange and anion exchange chromatography steps. At the sending unit, the product was eluted from Protein A with acetate buffer pH 3.2 and the product eluted from th column within the required range for the viral inactivation step. The eluate was pH adjusted to pH5.0 for the cation exchange step using 1 part product to 4 parts titrant. Prior to manufacture of the first GMP batch, a laboratory scale and pilot scale evaluation was performed at the receiving unit. During the laboratory scale evaluation at the receivin unit, two issues arose with the Protein A step in the purification process. A root cause analysis (Ishikawa diagram) was performed on the Protein A step. A number of items were highlighted for further investigation between the two units, relating to elution buffer and titrant preparation. During the transfer, only the names of the buffers were shared and not the preparation details. Two key differences were identified: - At the sending unit, the elution buffer and the titrant were prepared using the trihydrate salt, compared to use of the anhydrous salt at the receiving unit. - At the sending unit, the elution buffer was titrated with hydrochloric acid, compared to titration with acetate acid at the receiving unit which resulted in much higher buffering capacity. The challenges and the corresponding solutions for this technology transfer are summarized in Table 9.6. Table 9.7 summarizes the challenges and resolutions prior to the pilot scale batch. Table 9.7: Biologics Case Study \#3 - Challenges and Resolutions Result: Formulation of the elution buffer with the trihydrate salt and adjustment of the acetate concentration resulted in the eluted product pH achieving the required pH range for viral inactivation. Preparation of the titrant with the trihydrate salt and adjustment of the acetate concentration resulted in the expected volume of titrant being added for pH adjustment to pH5.0. What Worked: - Good communication between technical SMEs at sending and receiving units: the unexpected performance of the Protein A step and potential causes were flagged for the receiving unit, so details of buffer and titrant preparation could be compared quickly and adjusted as the laboratory scale evaluation was ongoing - Formulation of the elution buffer facilitated preparation at scale in the GMP facility Developing a Charter The charter can include: 1. Assumptions and constraints 2. Sites impacted (sending and receiving units) 3. Scope a) Includes overview of the project and business drivers b) Items that are out of scope are equally important to document in the charter 4. Objectives and technical success criteria 5. Roles and responsibilities 6. Technology transfer team 7. Governance 8. High level technology transfer plan2 9. Regulatory strategy 10. Change control procedure 11. Technology transfer completions and close out
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


