Question: 3 Closed System with Weight / Internal Energy U Gas at a pressure of p 1 = 0 . 4 MPa and a temperature of
Closed System with Weight Internal Energy U
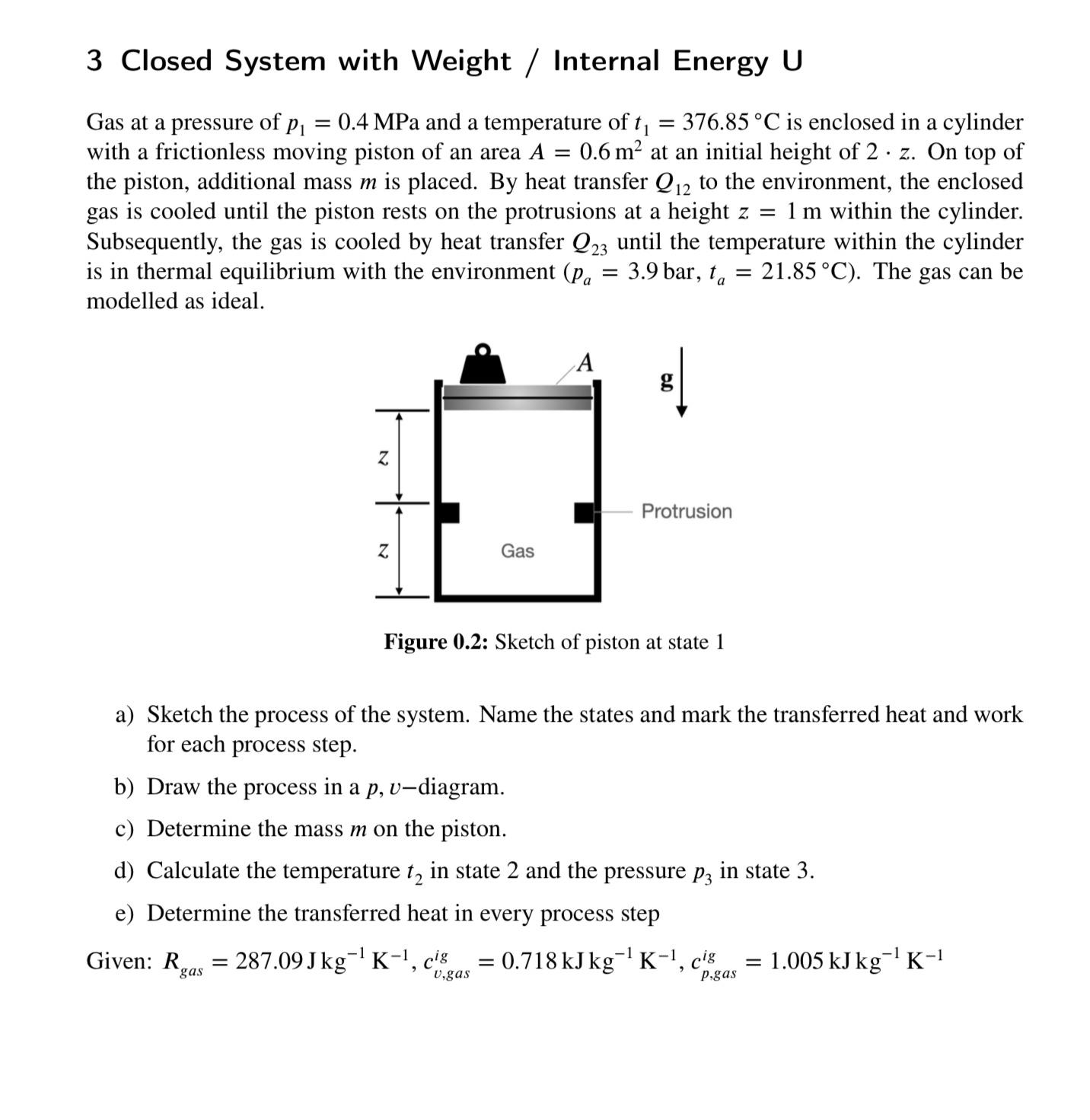
Gas at a pressure of MPa and a temperature of is enclosed in a cylinder with a frictionless moving piston of an area at an initial height of On top of the piston, additional mass is placed. By heat transfer to the environment, the enclosed gas is cooled until the piston rests on the protrusions at a height within the cylinder. Subsequently, the gas is cooled by heat transfer until the temperature within the cylinder is in thermal equilibrium with the environment The gas can be modelled as ideal.
Figure : Sketch of piston at state
a Sketch the process of the system. Name the states and mark the transferred heat and work for each process step.
b Draw the process in a diagram.
c Determine the mass on the piston.
d Calculate the temperature in state and the pressure in state
e Determine the transferred heat in every process step
Given:
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


