Question: 3. Complete the Energy Transition chart below (Use these equations and constants) hc 'photon 1 eV = 1.6 x 10-19 J h =6.63 x 10
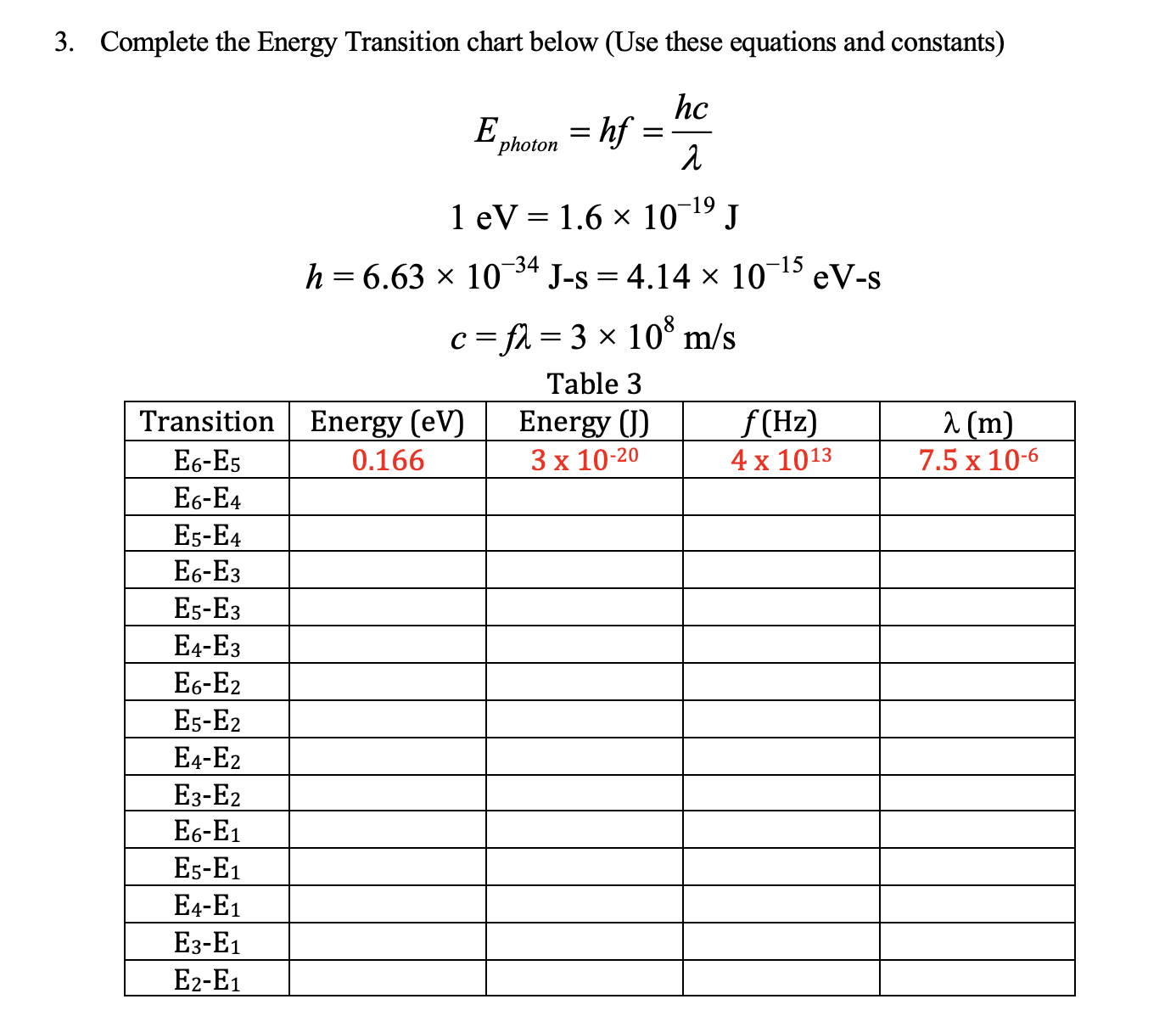
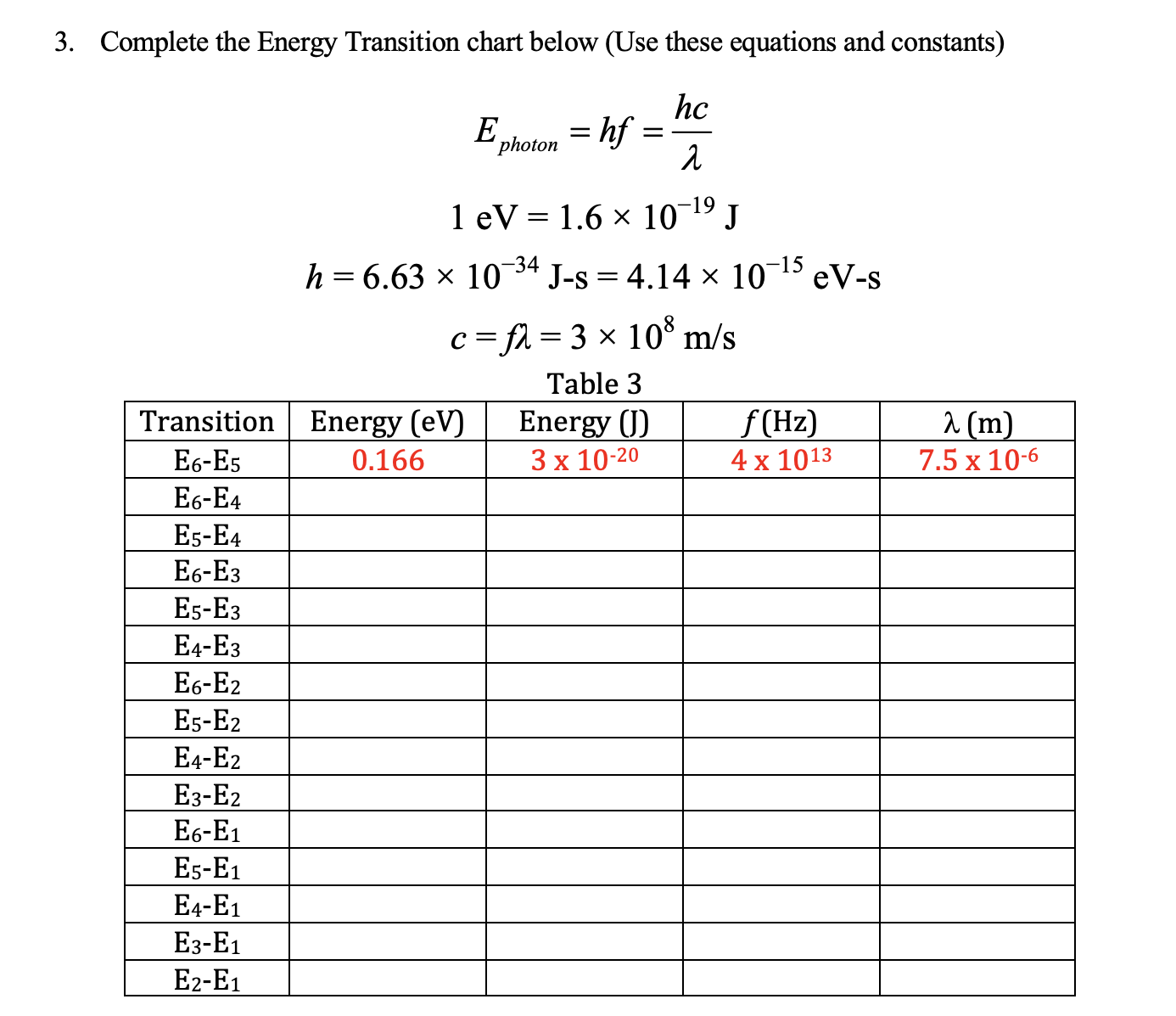
3. Complete the Energy Transition chart below (Use these equations and constants) hc 'photon 1 eV = 1.6 x 10-19 J h =6.63 x 10 34 J-s = 4.14 x 10 15 eV-s c =fl = 3 x 108 m/s Table 3 Transition Energy (ev) Energy () f (Hz) 2 (m E6-E5 0.166 3 x 10-20 4 x 1013 7.5 x 10-6 6-E4 E5-E4 E6-E3 E5-E3 E4-E3 E6-E2 E5-E2 E4-E2 E3-E2 E6-E1 E5-E1 E4-E1 E3-E1 E2-E1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


