Question: 3. Consider the same system as in problem 2 , but now the piston is thermally isolated (adiabatic), so that q=0. Sketch the approximate shape
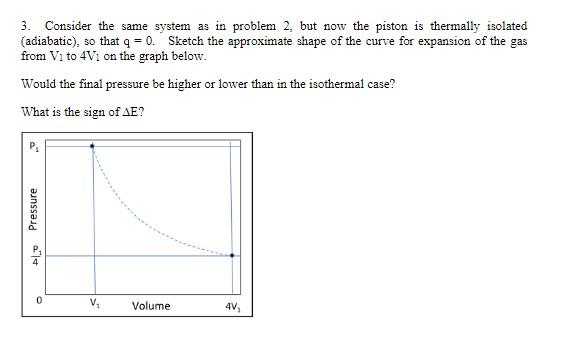
3. Consider the same system as in problem 2 , but now the piston is thermally isolated (adiabatic), so that q=0. Sketch the approximate shape of the curve for expansion of the gas from V1 to 4V1 on the graph below. Would the final pressure be higher or lower than in the isothermal case? What is the sign of E
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


