Question: 3. For various combination quantum numbers I, mi and ms, enter the appropriate information in the following table. 4. mi I ms Type of
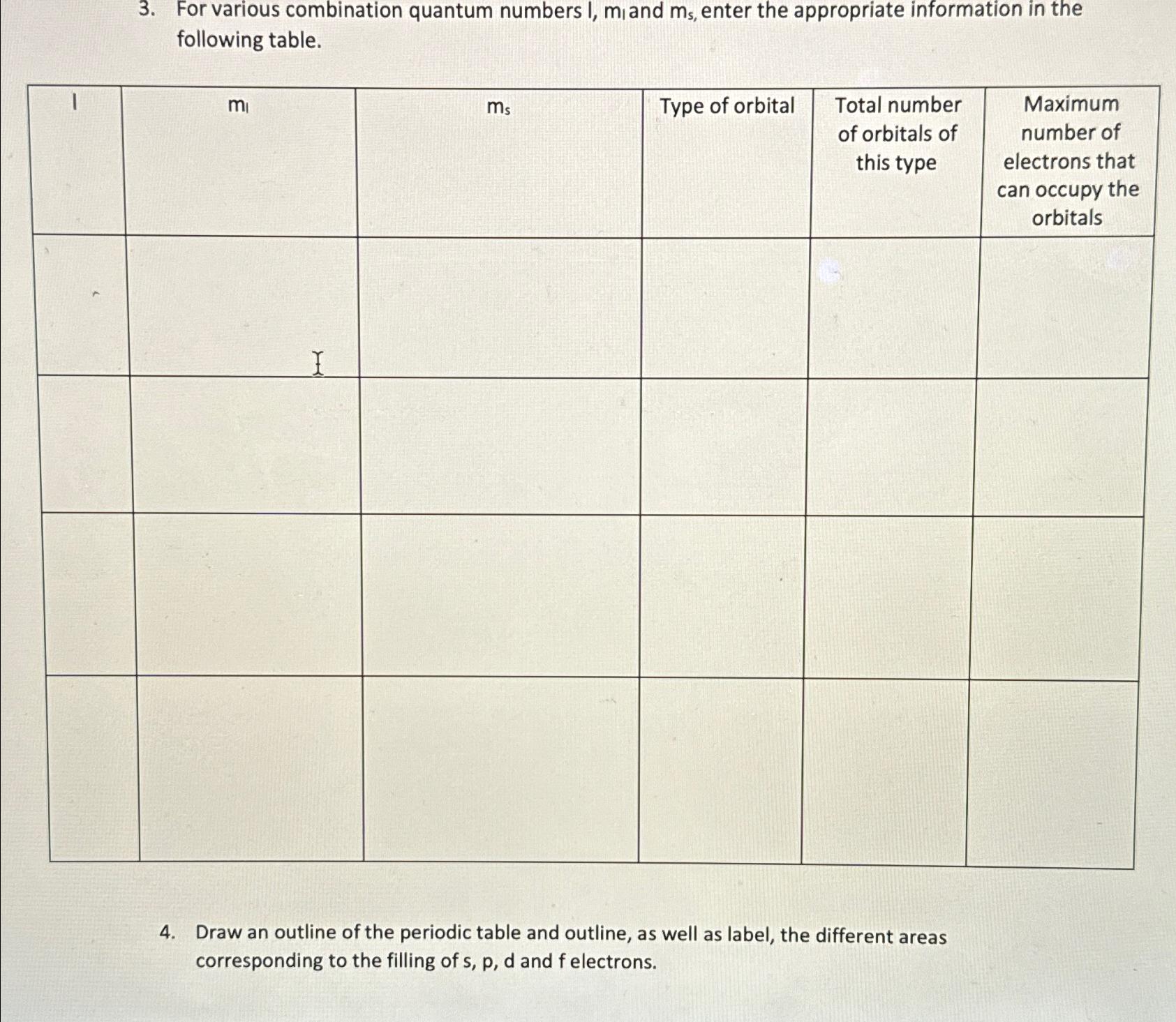
3. For various combination quantum numbers I, mi and ms, enter the appropriate information in the following table. 4. mi I ms Type of orbital Total number of orbitals of this type Draw an outline of the periodic table and outline, as well as label, the different areas corresponding to the filling of s, p, d and f electrons. Maximum number of electrons that can occupy the orbitals
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


