Question: (3) is the first question that says consider solution of pure water in equilibrium with mineral stronzianite (SrCO3(s)). SrCO3(s) = Sr2+(aq) + CO32-(aq) Calculate
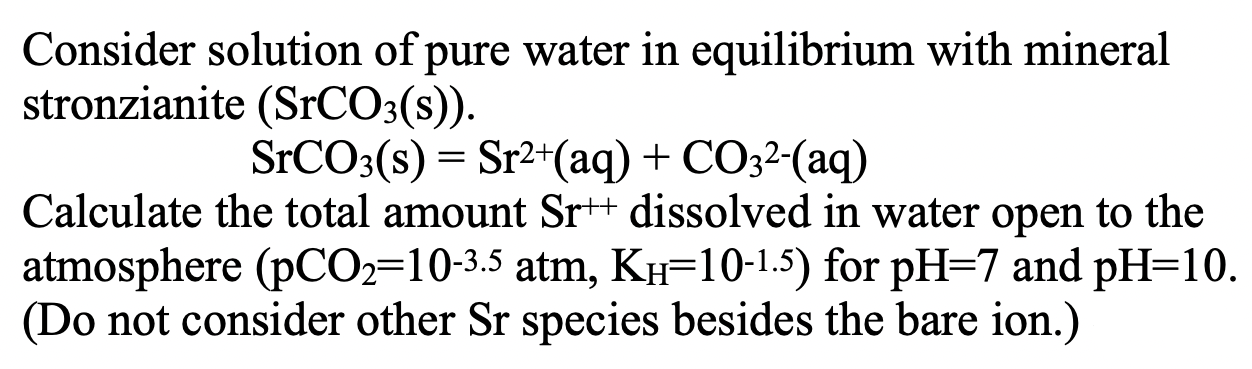
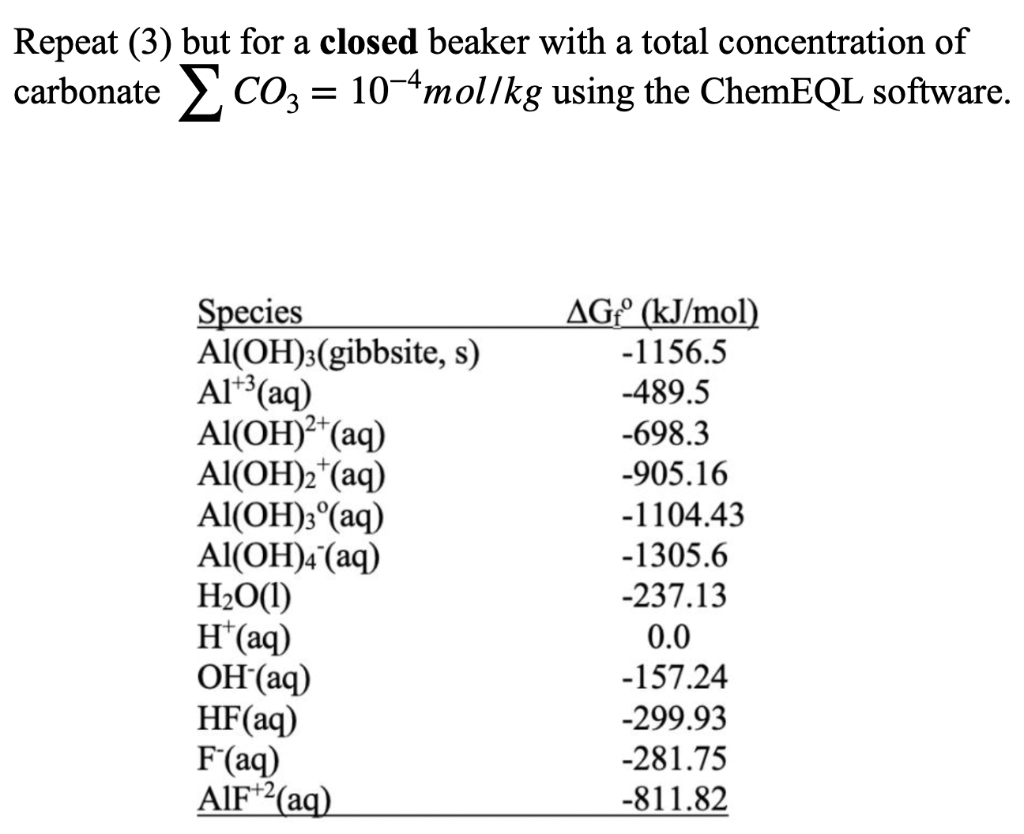
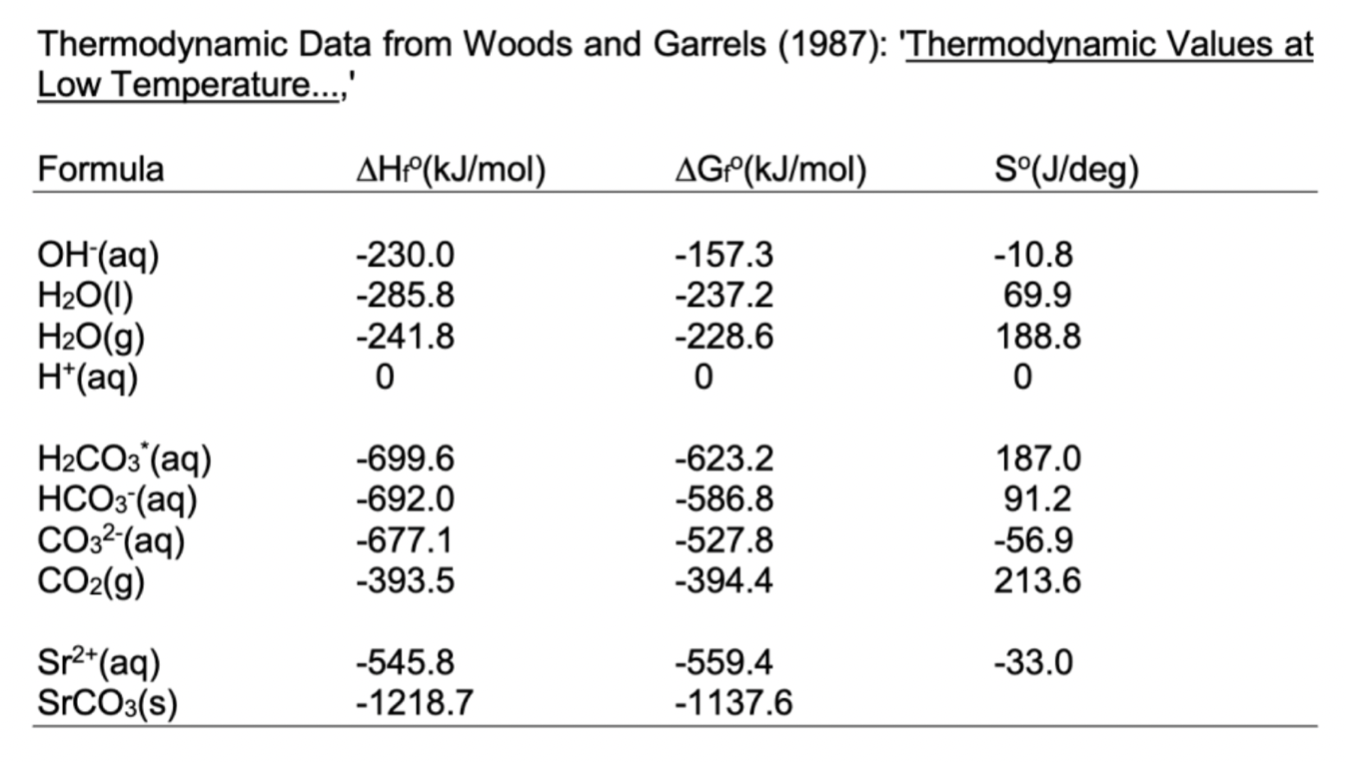
= Consider solution of pure water in equilibrium with mineral stronzianite (SrCO3(s)). SrCO3(s) = Sr2+(aq) + CO32-(aq) Calculate the total amount Sr++ dissolved in water open to the atmosphere (PCO2=10-3.5 atm, Kh=10-1.5) for pH=7 and pH=10. (Do not consider other Sr species besides the bare ion.) a a Repeat (3) but for a closed beaker with a total concentration of carbonate coz = 10-4mol/kg using the ChemEQL software. = Species Al(OH)3(gibbsite, s) Al'(aq) Al(OH)2+(aq) Al(OH)2 (aq) Al(OH)3(aq) Al(OH)4 (aq) H2O(1) H(aq) OH(aq) HF(aq) F'(aq) AlF+2(aq) AG (kJ/mol) -1156.5 -489.5 -698.3 -905.16 -1104.43 -1305.6 -237.13 0.0 -157.24 -299.93 -281.75 -811.82 Thermodynamic Data from Woods and Garrels (1987): 'Thermodynamic Values at Low Temperature...,' Formula AH(kJ/mol) AGP(kJ/mol) S(J/deg) OH(aq) H2O(0) H2O(g) H*(aq) -230.0 -285.8 -241.8 0 -157.3 -237.2 -228.6 0 -10.8 69.9 188.8 o H2CO3(aq) HCO3(aq) CO32-(aq) CO2(g) -699.6 -692.0 -677.1 -393.5 -623.2 -586.8 -527.8 -394.4 187.0 91.2 -56.9 213.6 -33.0 Sr2+(aq) SrCO3(s) -545.8 -1218.7 -559.4 -1137.6 = Consider solution of pure water in equilibrium with mineral stronzianite (SrCO3(s)). SrCO3(s) = Sr2+(aq) + CO32-(aq) Calculate the total amount Sr++ dissolved in water open to the atmosphere (PCO2=10-3.5 atm, Kh=10-1.5) for pH=7 and pH=10. (Do not consider other Sr species besides the bare ion.) a a Repeat (3) but for a closed beaker with a total concentration of carbonate coz = 10-4mol/kg using the ChemEQL software. = Species Al(OH)3(gibbsite, s) Al'(aq) Al(OH)2+(aq) Al(OH)2 (aq) Al(OH)3(aq) Al(OH)4 (aq) H2O(1) H(aq) OH(aq) HF(aq) F'(aq) AlF+2(aq) AG (kJ/mol) -1156.5 -489.5 -698.3 -905.16 -1104.43 -1305.6 -237.13 0.0 -157.24 -299.93 -281.75 -811.82 Thermodynamic Data from Woods and Garrels (1987): 'Thermodynamic Values at Low Temperature...,' Formula AH(kJ/mol) AGP(kJ/mol) S(J/deg) OH(aq) H2O(0) H2O(g) H*(aq) -230.0 -285.8 -241.8 0 -157.3 -237.2 -228.6 0 -10.8 69.9 188.8 o H2CO3(aq) HCO3(aq) CO32-(aq) CO2(g) -699.6 -692.0 -677.1 -393.5 -623.2 -586.8 -527.8 -394.4 187.0 91.2 -56.9 213.6 -33.0 Sr2+(aq) SrCO3(s) -545.8 -1218.7 -559.4 -1137.6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


