Question: 3.) Please explain the bonding in ethylene. H H A typical example how to go about this is as follows for CH, (methane): H-C +
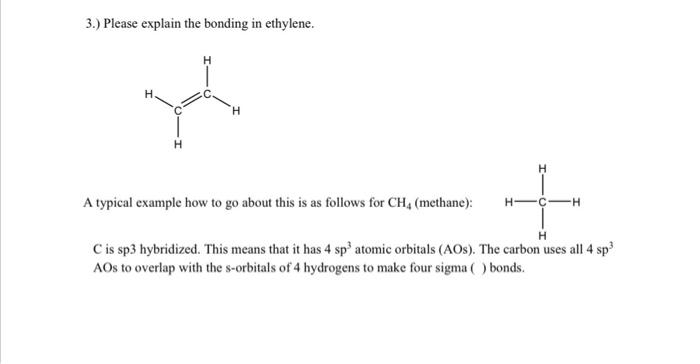
3.) Please explain the bonding in ethylene. H H A typical example how to go about this is as follows for CH, (methane): H-C + H Cis sp3 hybridized. This means that it has 4 sp' atomic orbitals (AOS). The carbon uses all 4 sp? AOs to overlap with the s-orbitals of 4 hydrogens to make four sigma) bonds
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


