Question: 3 points Calculate the van't Hoff factor, i, for an aqueous sodium hydroxide solution made from dissolving 7.20g of it in 50.00g of water. The
3 points\ Calculate the van't Hoff factor, i, for an aqueous sodium hydroxide solution made from dissolving
7.20gof it in
50.00gof water. The experimental freezing point for this solution is
-10.20\\\\deg C. The freezing point constant for water is
1.86(\\\\deg Ckg)/(mol). Assume water freezes at
0.0\\\\deg C.
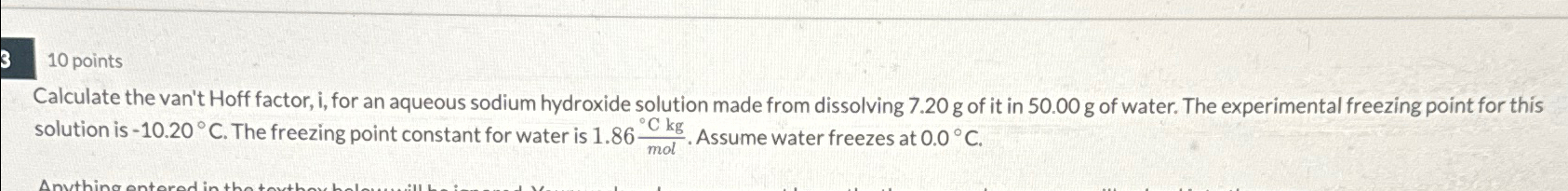
Calculate the van't Hoff factor, i, for an aqueous sodium hydroxide solution made from dissolving 7.20g of it in 50.00g of water. The experimental freezing point for this solution is 10.20C. The freezing point constant for water is 1.86molCkg. Assume water freezes at 0.0C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


