Question: 3. Repeat the calculations in Example 3.8, with same mass input for the Level I model, for benzene, lindane, and phenanthrene. See Table 2.3 for

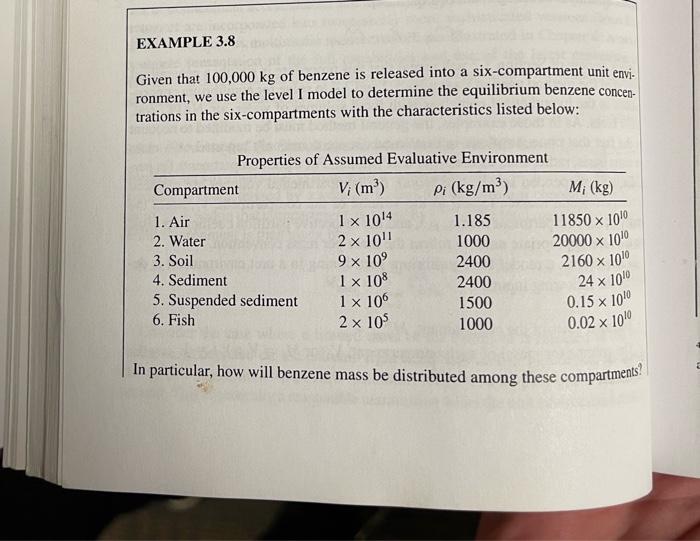
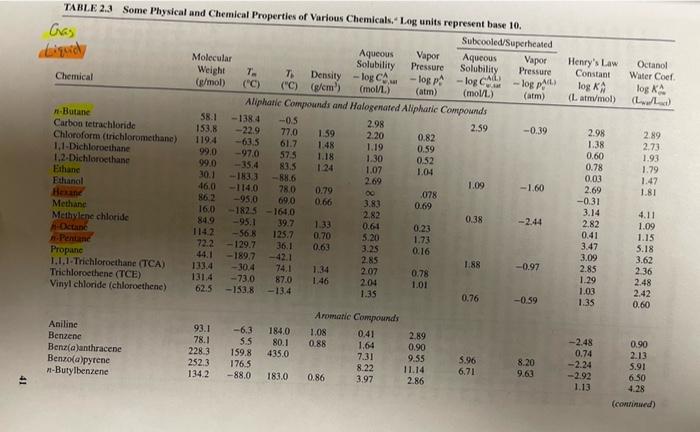
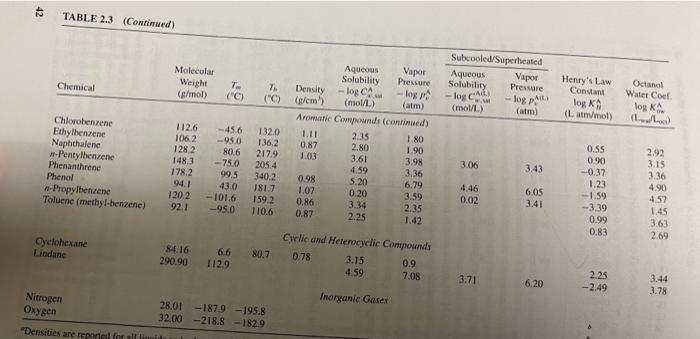
3. Repeat the calculations in Example 3.8, with same mass input for the Level I model, for benzene, lindane, and phenanthrene. See Table 2.3 for basic chemical properties and derive any partition coefficients that you need that are not presented in the table using the relationships presented in Example 3.8 or elsewhere in Chapter 3. Use this relationship to estimate Kx Ker=0.41* Key 1) Do calculations for three chemicals in Excel 2) Discuss how the results you obtain differ among these three chemicals 3) Given your results, does the assumption in Problem 2 above-that volatilization of lindane to the air can be ignored - seem reasonable? 4) What are the major properties of these chemicals responsible for these differences? EXAMPLE 3.8 Given that 100,000 kg of benzene is released into a six-compartment unit envi- ronment, we use the level I model to determine the equilibrium benzene concen- trations in the six-compartments with the characteristics listed below: M; (kg) X Properties of Assumed Evaluative Environment Compartment Vi (m) Pi (kg/m3) 1. Air I x 1014 1.185 11850 x 100 2. Water 2 x 101 1000 20000 x 100 3. Soil 9 x 109 2400 2160 x 100 4. Sediment 1 x 108 2400 5. Suspended sediment 1 x 106 1500 0.15 x 10 X 6. Fish 2 x 10 1000 24 x 100 0.02 x 10 In particular, how will benzene mass be distributed among these compartments? - Tog C. Henry's Law Constant log KA (Lammol) Octanol Water Coef log A ( LL) TABLE 2.3 Some Physical and Chemical Properties of Various Chemicals. Log units represent base 10. Gas Subcooled/Superheated Aqueous Molecular Vapor Aqueous Vapor Solubility Pressure Weight Pressure Solubility 7. 7 Chemical Density (g/mol) - logp-log Call ("C) (C) (@cm) - logo (moll) (atm) (mol/L) (atm) Aliphatic Compounds and Halogenated Aliphatic Compounds -Butane 58.1 - 1384 -0.5 Carbon tetrachloride 2.98 153.8 2.59 -0.39 -22.9 77.0 1.59 Chloroform (trichloromethane) 2.20 0.82 1194 -63.5 61.7 1.48 1.19 1,1-Dichloroethane 0.59 99.0 -97.0 575 1.2-Dichloroethane 1.18 1.30 0.52 99.0 -35,4 83.5 Ethane 124 1.07 1.04 30.1 183.3 -88.6 2.69 Ethanol 1.09 46,0 -1.60 -114.0 28.0 Heun 0.79 00 078 86,2 -95,0 69,0 0.66 3.83 Methane 0.69 160 -1825-164.0 2.82 Methylene chloride 0.38 -2.44 849 -95.1 39.7 1.33 -Octane 0.61 0.23 1142 -56,8 125.7 0.70 Pentane 5.20 1.73 72.2 - 129.7 36.1 0.63 3.25 0.16 Propane 44.1 - 189.7 -42.1 1.1.1-Trichloroethane (TCA) 2.85 1.88 133.4 -0.97 -30.4 74.1 1.34 Trichloroethene (TCE) 2.07 0.78 1314 -73.0 87.0 1.46 2.04 1.01 Vinyl chloride (chloroethene) 62.5 -153.8 -13.4 1.35 0.76 -0.59 Aromatic Compounds Aniline 93.1 -6.3 184.0 1.08 0.41 Benzene 2.89 78.1 5.5 80.1 0.88 1.64 Benz(a)anthracene 0.90 228.3 159.8 435.0 7.31 9.55 Benzo(a)pyrene 5.96 8.20 252.3 176.5 8.22 11.14 6.71 -Butylbenzene 1342 9.63 -88.0 183.0 0.86 3.97 2.86 2.98 1.38 0.60 0.78 0.03 2.69 -0.31 2.89 2.73 1.93 1.79 1.47 1.81 3.14 2.82 0.41 3.47 3.09 2.85 1.29 1.03 1.35 4.11 1.09 1.15 5.18 3.62 2 36 2.48 2.42 0.60 -2.48 0.74 -2.24 -2.92 1.13 0.90 2.13 5.91 6.50 4.28 (continued) ID 42 TABLE 2.3 (Continued) Chemical Molecular Weight (g/mol) 7 ("C) 7 ("C) Subcooled/Superheated Aqueous Vapor Solubility Pressure - log pita (mol/L) (atm) w logo Henry's Law Constant log KA (Lanmol) Octanol Water Cool log KA (...) Chlorobenzene Ethylbenzene Naphthalene -Pentylbenzene Phenanthrene Phenol -Propylbenzene Toluene (methyl-benzene) 112.6 106,2 1282 148.3 178.2 94.1 120.2 92.1 -45.6 -95.0 80,6 -75.0 99.5 43.0 -101.6 -95.0 Aqueous Vapor Solubility Pressure Density-log C - logp 4 g/cm) (mol/L) (atm) Aromatic Compounds (continued) 1.11 2.35 1.80 0.87 2.80 1.90 1.03 3.61 3.98 4.59 3.36 0.98 5.20 1.07 0.20 3.59 0,86 3.34 2.35 0.87 1.42 132.0 136,2 217.9 205.4 340.2 181.7 1592 110.6 3.06 3.43 6.79 4.46 0.02 6.05 0.55 0.90 -0.37 1.23 -1.59 -3.39 0.99 0.83 2.92 3.15 3.36 4.90 452 1.45 3.63 269 341 2.25 Cyclohexane Lindane 84.16 290.90 6.6 112.9 80.7 Cyclic and Heterocyclic Compounds 0.78 3.15 0.9 4.59 7.08 3.71 6.20 2.25 -2.49 3.44 3.78 Inorganic Gases Nitrogen Oxygen "Densities are reported for all li 28.01 -1879-195.8 32.00-218.8 -182.9
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


