Question: 3 slightly less than 5+ 5+ equal to greater than 2 6+ 7+ less than nearly 6+ slightly greater than 5+ nearly zero less
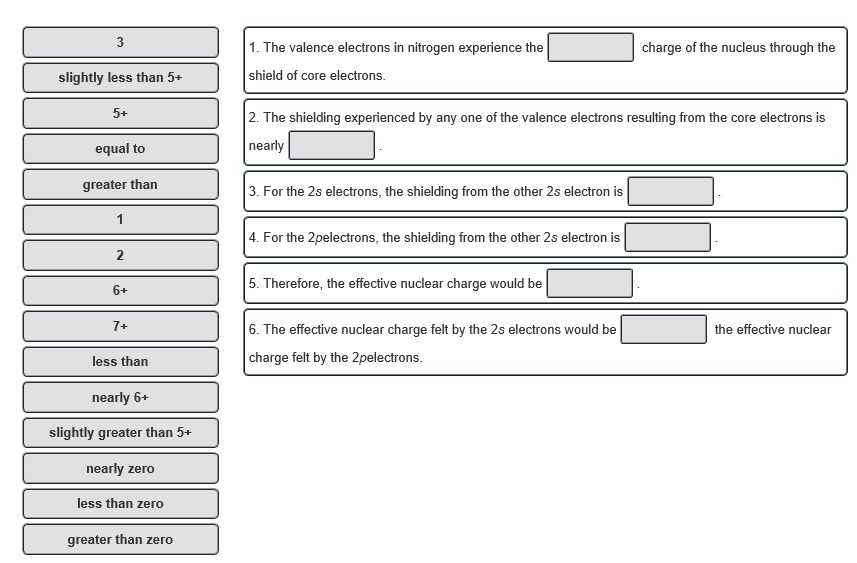
3 slightly less than 5+ 5+ equal to greater than 2 6+ 7+ less than nearly 6+ slightly greater than 5+ nearly zero less than zero greater than zero 1. The valence electrons in nitrogen experience the shield of core electrons. 2. The shielding experienced by any one of the valence electrons resulting from the core electrons is nearly 3. For the 2s electrons, the shielding from the other 2s electron is 4. For the 2pelectrons, the shielding from the other 2s electron is 5. Therefore, the effective nuclear charge would be charge of the nucleus through the 6. The effective nuclear charge felt by the 2s electrons would be charge felt by the 2pelectrons. the effective nuclear
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it
Answer 1 The valence electrons in nitrogen experience the charge of the nucleus through the slightly ... View full answer

Get step-by-step solutions from verified subject matter experts


