Question: 3 STEP CONVERSIONS Question 6 (1 paint Place the steps below in the proper order of first to last for this grams-to-grams Stoichiometry probleme Given
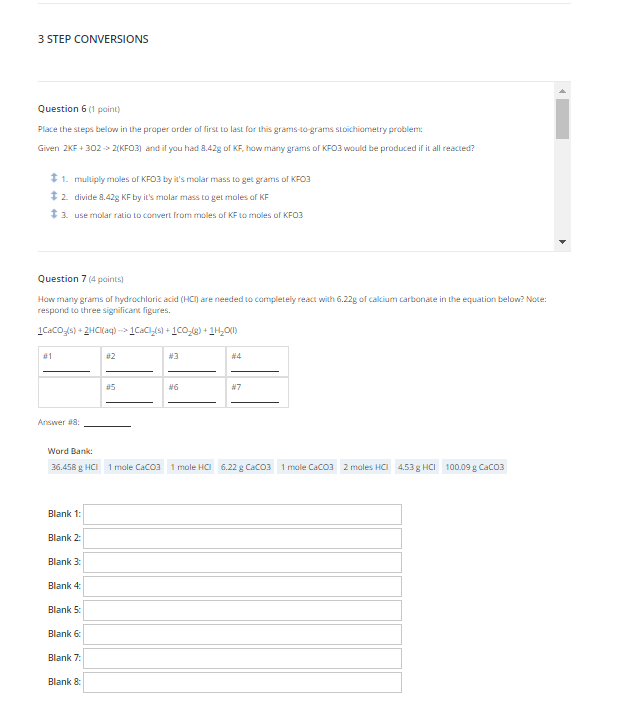
3 STEP CONVERSIONS Question 6 (1 paint Place the steps below in the proper order of first to last for this grams-to-grams Stoichiometry probleme Given ZKF+ 302 > 2/KF03) and if you had 2.42g of KF, how many grams of KFO3 would be produced if it all reacted? 1. Multiply moles of KFO3 by it's molar mass to get grams of KFO3 2 divide 8.429 KF by it's molar mass to get moles of KF 13. use molar ratio to convert from moles of KF to moles of KFO3 Question 7 (4 points) How many grams of hydrochloric acid (HC) are needed to completely react with 6.22g of calcium carbonate in the equation below? Note: respond to three significant figures. 1CaCO3(s) + 2HCl(aq) -> 1CACI_() +100_(8) +11201) #1 #2 #3 3 #4 #6 #7 Answer : Word Bank: 36.458 g HCI 1 male Cacoa 1 mole HCI 6.22 g CaCo3 1 mole Cacoa 2 males HCI 4.53 g HCI 100.09 g Caco3 Blank 1: Blank 2: Blank 3: Blank 4: Blank 5: Blank 6: Blank 7 Blank &
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


