Question: 3. The Stefan-Maxwell equations are used to describe multicomponent diffusion in an ideal gas mixture. A readable account of the derivation can be found in
3. The Stefan-Maxwell equations are used to describe multicomponent diffusion in an ideal gas mixture. A readable account of the derivation can be found in Taylor and Krishna
3. The Stefan-Maxwell equations are used to describe multicomponent diffusion in an ideal gas mixture. A readable account of the derivation can be found in Taylor and Krishna
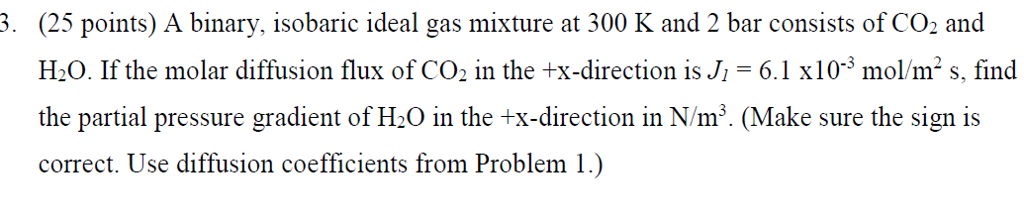
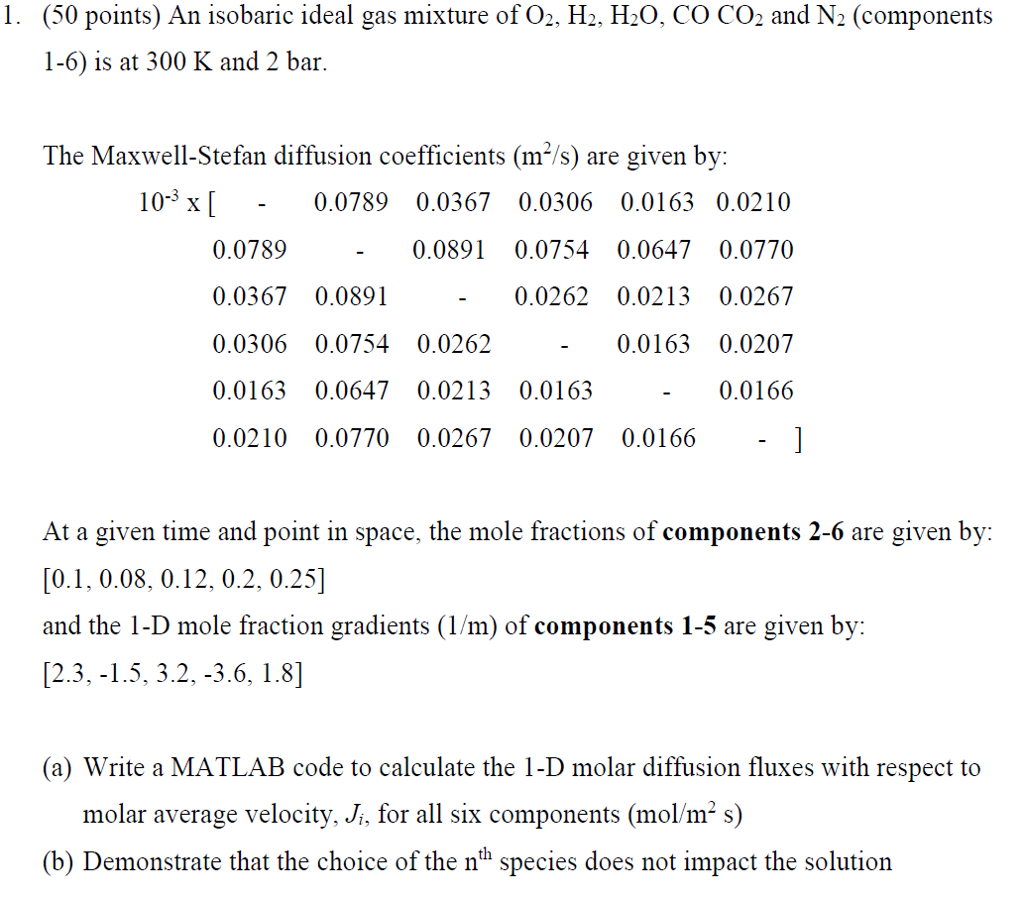
. (25 points) A binary, isobaric ideal gas mixture at 300 K and 2 bar consists of CO2 and H20. If the molar diffusion flux of CO2 in the +x-direction is J 6.1 x10-3mo/m2s, find the partial pressure gradient of H20 in the +x-direction in N/m3. (Make sure the sign is correct. Use diffusion coefficients from Problem 1.) . (25 points) A binary, isobaric ideal gas mixture at 300 K and 2 bar consists of CO2 and H20. If the molar diffusion flux of CO2 in the +x-direction is J 6.1 x10-3mo/m2s, find the partial pressure gradient of H20 in the +x-direction in N/m3. (Make sure the sign is correct. Use diffusion coefficients from Problem 1.)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


