Question: 3. Try to do the example lecture question, and ask any questions you have: For a Na+Clion pair, attractive and repulsive energies EA and ER,
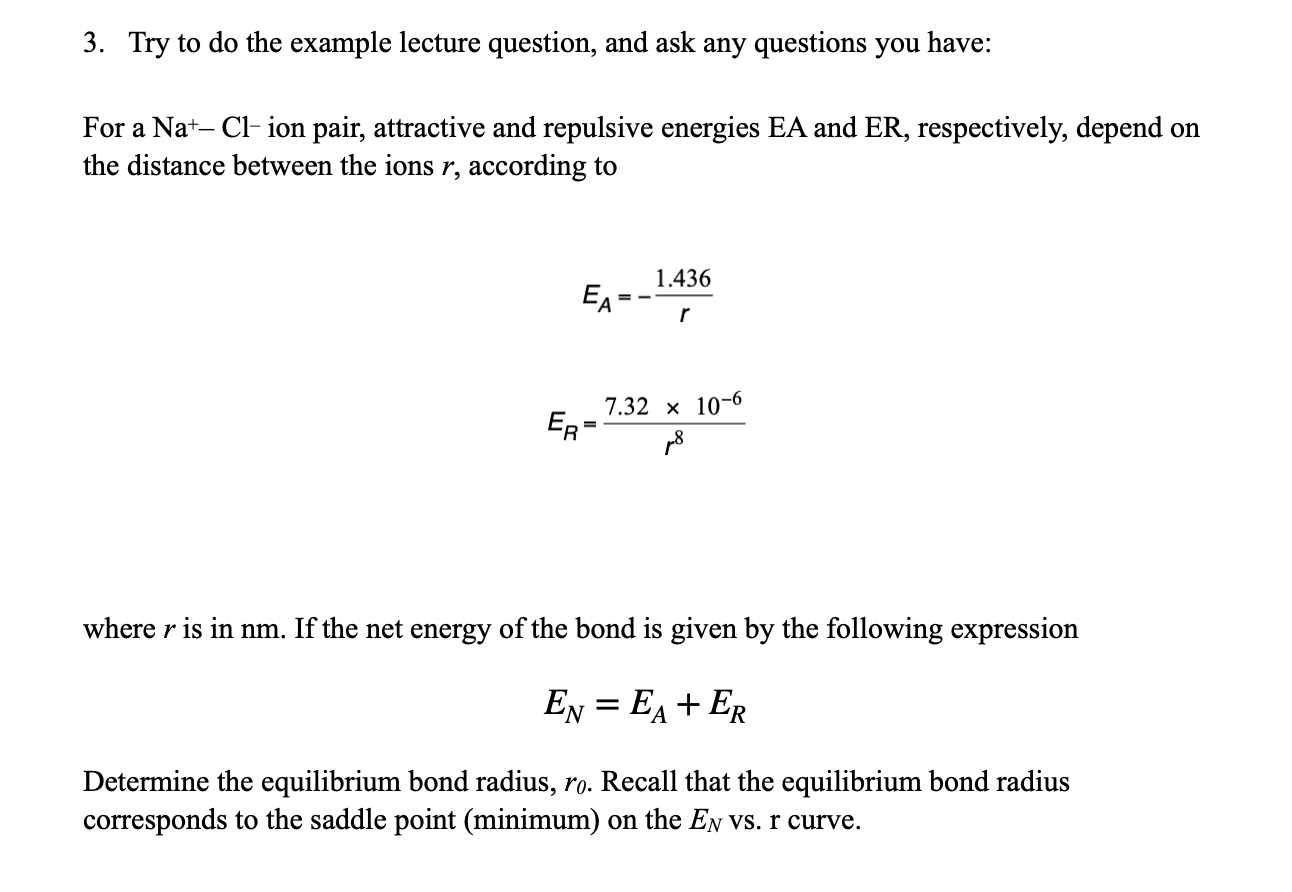
3. Try to do the example lecture question, and ask any questions you have: For a Na+Clion pair, attractive and repulsive energies EA and ER, respectively, depend on the distance between the ions r, according to EA=r1.436ER=r87.32106 where r is in nm. If the net energy of the bond is given by the following expression EN=EA+ER Determine the equilibrium bond radius, r0. Recall that the equilibrium bond radius corresponds to the saddle point (minimum) on the EN vs. r curve
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


