Question: (30 points) ApH electrode is constructed using an indicator electrode consisting of an Ag/AgCl internal reference electrode and a pH-sensitive glass membrane together with an
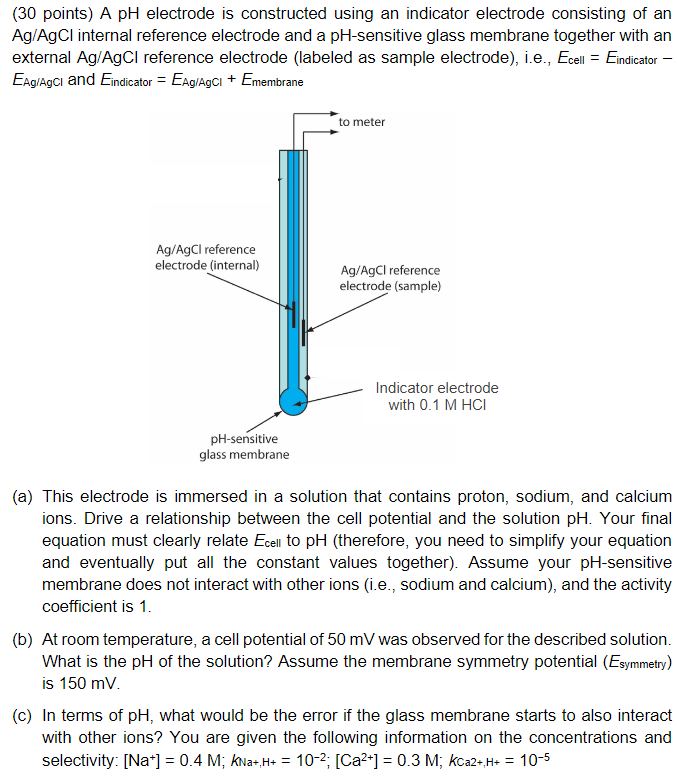
(30 points) ApH electrode is constructed using an indicator electrode consisting of an Ag/AgCl internal reference electrode and a pH-sensitive glass membrane together with an external Ag/AgCl reference electrode (labeled as sample electrode), i.e., Ecell=Eindicator EAg/AgCl and Eindicator=EAg/AgCl+Emembrane (a) This electrode is immersed in a solution that contains proton, sodium, and calcium ions. Drive a relationship between the cell potential and the solution pH. Your final equation must clearly relate Ecell to pH (therefore, you need to simplify your equation and eventually put all the constant values together). Assume your pH-sensitive membrane does not interact with other ions (i.e., sodium and calcium), and the activity coefficient is 1. (b) At room temperature, a cell potential of 50mV was observed for the described solution. What is the pH of the solution? Assume the membrane symmetry potential ( Esymmetry) is 150mV. (c) In terms of pH, what would be the error if the glass membrane starts to also interact with other ions? You are given the following information on the concentrations and selectivity: [Na+]=0.4M;kNa+,H+=102;[Ca2+]=0.3M;kCaa+,H=105
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


