Question: 3-10 A general rate expression for the ineversible reaction A + B + C can be written as: rc = kc cc? Use a spreadsheet
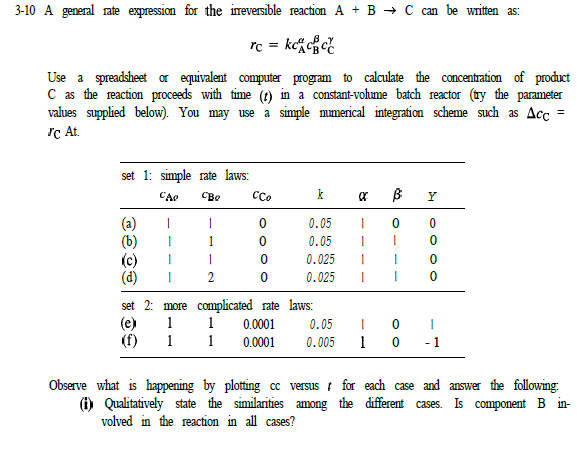

3-10 A general rate expression for the ineversible reaction A + B + C can be written as: rc = kc cc? Use a spreadsheet or equivalent computer program to calculate the concentration of product C as the reaction proceeds with time (t) in a constant-volume batch reactor (ty the parameter values supplied below). You may use a simple numerical integration scheme such as Acc Tc At set 1: simple rate laws: CA CCO k R 8 B Y 0 0 1 1 1 1 (a) 1 0 0.05 0 0.05 (c) 1 0 0.025 (d) 2 0 0.025 set 2: more complicated rate laws: (e) 1 1 0.0001 0.05 1 1 0.0001 0.005 | 1 1 0 0 1 -1 Observe what is happening by plotting cc versus for each case and answer the following. (i) Qualitatively state the similarities among the different cases. Is component B in- volved in the reaction in all cases? () By graphical means, find the time required to reach 20%, 50%, and 90% of the ultimate concentration for each case. (ii) Compare results of a) and 6). () and ). ) and (d), @) and e), and (a) and (f). Explam differences any
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


