Question: 3.134 As shown in Fig. P3.134, a rigid tank initially contains 3 kg of carbon dioxide (CO2) at 500 kPa. The tank is connected
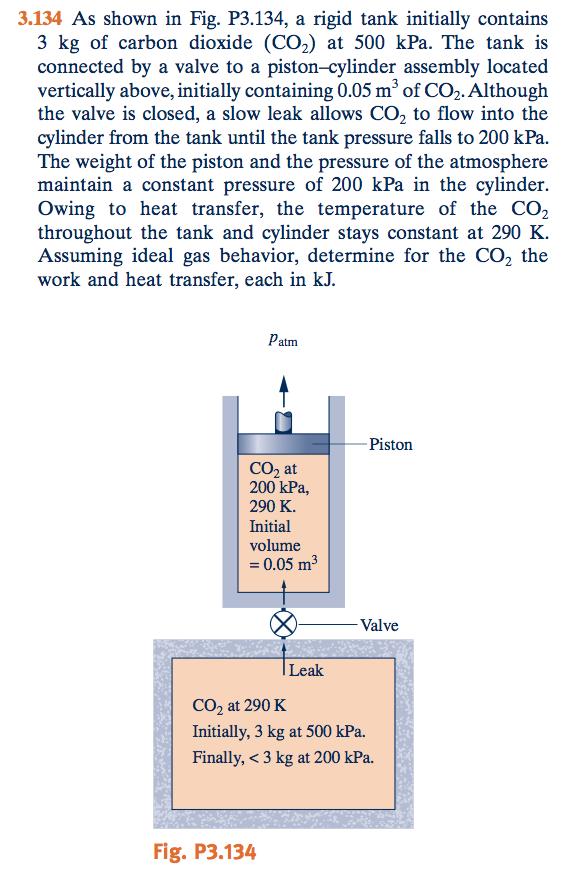
3.134 As shown in Fig. P3.134, a rigid tank initially contains 3 kg of carbon dioxide (CO2) at 500 kPa. The tank is connected by a valve to a piston-cylinder assembly located vertically above, initially containing 0.05 m of CO2.Although the valve is closed, a slow leak allows CO2 to flow into the cylinder from the tank until the tank pressure falls to 200 kPa The weight of the piston and the pressure of the atmosphere maintain a constant pressure of 200 kPa in the cylinder. Owing to heat transfer, the temperature of the CO2 throughout the tank and cylinder stays constant at 290 K Assuming ideal gas behavior, determine for the CO2 the work and heat transfer, each in kJ. Patm Piston CO2 at 200 kPa, 290 K Initial volume 0.05 m3 Valve Leak CO2 at 290 K Initially, 3 kg at 500 kPa. Finally, < 3 kg at 200 kPa Fig. P3.134
Step by Step Solution
3.31 Rating (154 Votes )
There are 3 Steps involved in it
To solve this problem we need to determine the work and heat transfer for the carbon dioxide CO usin... View full answer

Get step-by-step solutions from verified subject matter experts


