Question: 3.16 Poly(propylene fumerate) is a promising polymer for implants for orthopedic applications. It is synthesized in a methylene chloride solvent using a zinc chloride (Z.nCl2)
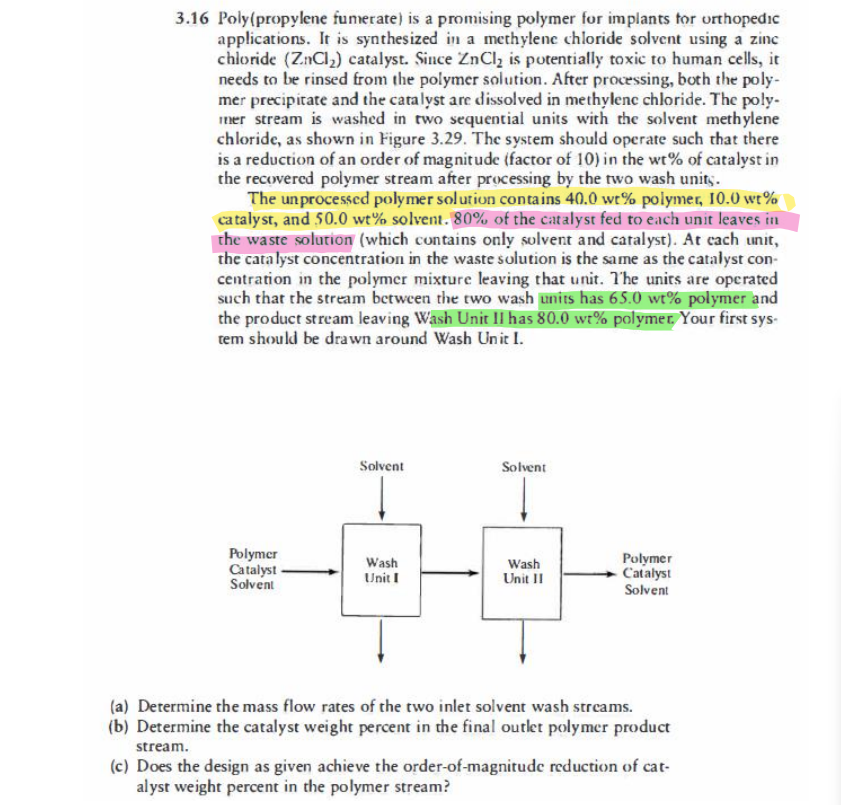
3.16 Poly(propylene fumerate) is a promising polymer for implants for orthopedic applications. It is synthesized in a methylene chloride solvent using a zinc chloride (Z.nCl2) catalyst. Since ZnCly is potentially toxic to human cells, it needs to be rinsed from the polymer solution. After processing, both the poly- mer precipitate and the catalyst are dissolved in methylene chloride. The poly- mer stream is washed in two sequential units with the solvent methylene chloride, as shown in Figure 3.29. The system should operate such that there is a reduction of an order of magnitude (factor of 10) in the wt% of catalyst in the recovered polymer stream after processing by the two wash unity. The unprocessed polymer solution contains 40.0 wt% polymer, 10.0 wt % catalyst, and 50.0 wt% solvent. 80% of the catalyst fed to each unit leaves in the waste solution (which contains only solvent and catalyst). At cach unit, the catalyst concentration in the waste solution is the same as the catalyst con- centration in the polymer mixture leaving that unit. The units are operated such that the stream between the two wash units has 65.0 wt% polymer and the product stream leaving Wash Unit II has 80.0 wt% polymer. Your first sys- tem should be drawn around Wash Unit I. Solvent Solvent Polymer Catalyst Wash Wash Polymer Catalyst Solvent Unit I Unit II Solvent (a) Determine the mass flow rates of the two inlet solvent wash streams. (b) Determine the catalyst weight percent in the final outlet polymer product stream. (c) Does the design as given achieve the order-of-magnitude reduction of cat- alyst weight percent in the polymer stream
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


