Question: 3. Predict which test or tests will be positive for each of the compounds listed in the left-hand column, Mark (+) to indicate a
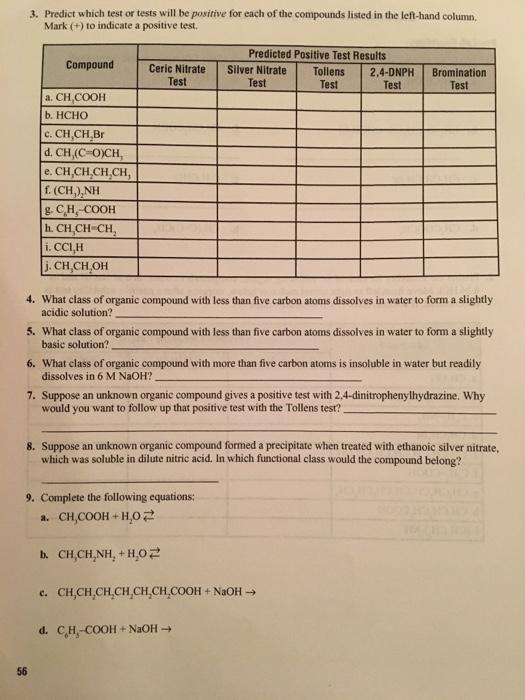
3. Predict which test or tests will be positive for each of the compounds listed in the left-hand column, Mark (+) to indicate a positive test, Predicted Positive Test Results Compound Ceric Nitrate Test Silver Nitrate Test Tollens 2,4-DNPH Test Bromination Test Test a. CH,COOH b. HCHO c. CH,CH Br d. CH,(C-O)CH, e. CH,CH,CH,CH, f(CH,),NH g. CH, COOH h. CH,CH-CH, i. CCI,H j.CH,CH,OH 4. What class of organic compound with less than five carbon atoms dissolves in water to form a slightly acidic solution? 5. What class of organic compound with less than five carbon atoms dissolves in water to form a slightly basic solution? 6. What class of organic compound with more than five carbon atoms is insoluble in water but readily dissolves in 6 M NaOH? 7. Suppose an unknown organic compound gives a positive test with 2,4-dinitrophenylhydrazine. Why would you want to follow up that positive test with the Tollens test? 8. Suppose an unknown organic compound formed a precipitate when treated with ethanoic silver nitrate, which was soluble in dilute nitric acid. In which functional class would the compound belong? 9. Complete the following equations: a. CH,COOH + H,0 2 b. CH,CH,NH, + H02 c. CH,CH,CH,CH,CH,CH,COOH + NaOH d. CH,-COOH + NaOH - 56
Step by Step Solution
3.42 Rating (146 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


