Question: 33. Consider the reaction: MISSED THIS? Read Section 15.2; Watch KCV 15.2, IWE 15.1 H2(g)+Br2(g)2HBr(g) The graph shows the concentration of Br2 as a function
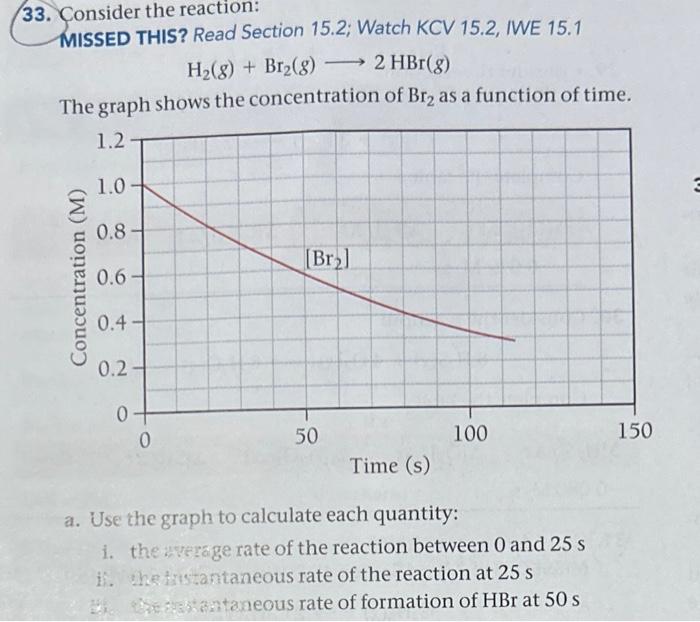
33. Consider the reaction: MISSED THIS? Read Section 15.2; Watch KCV 15.2, IWE 15.1 H2(g)+Br2(g)2HBr(g) The graph shows the concentration of Br2 as a function of time. a. Use the graph to calculate each quantity: i. the arerage rate of the reaction between 0 and 25s i. the tristantaneous rate of the reaction at 25s
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


