Question: 35 kg solid Lead is heated from -30C to 45C at atmospheric pressure. Assuming that there is no change in the volume of the
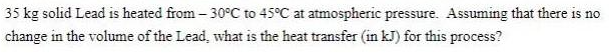
35 kg solid Lead is heated from -30C to 45C at atmospheric pressure. Assuming that there is no change in the volume of the Lead, what is the heat transfer (in kJ) for this process?
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


