Question: 38 Question (1 Point) Part 2 A + 2 B 2 C+D What is the liquid phase concentration of B as a function of initial
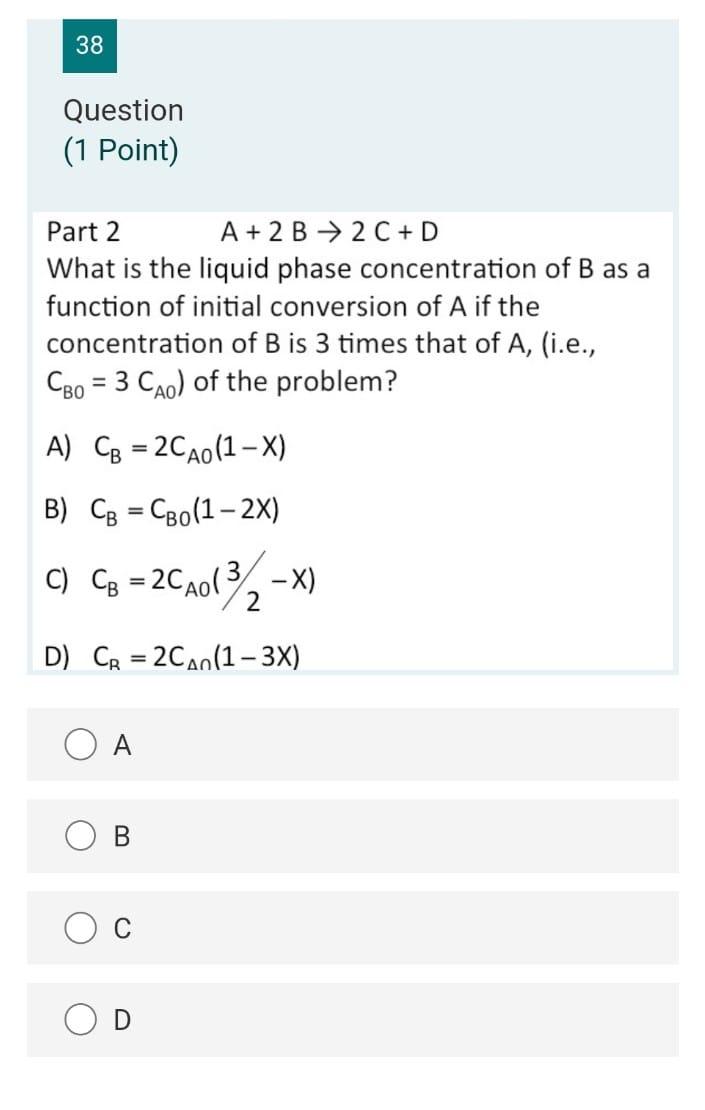



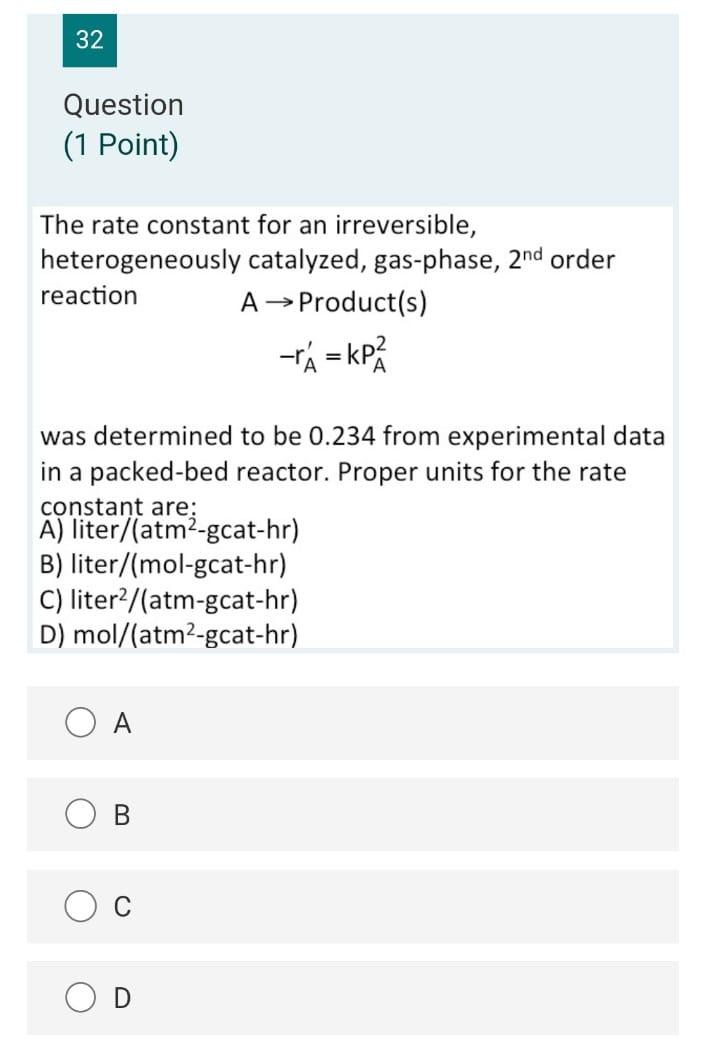
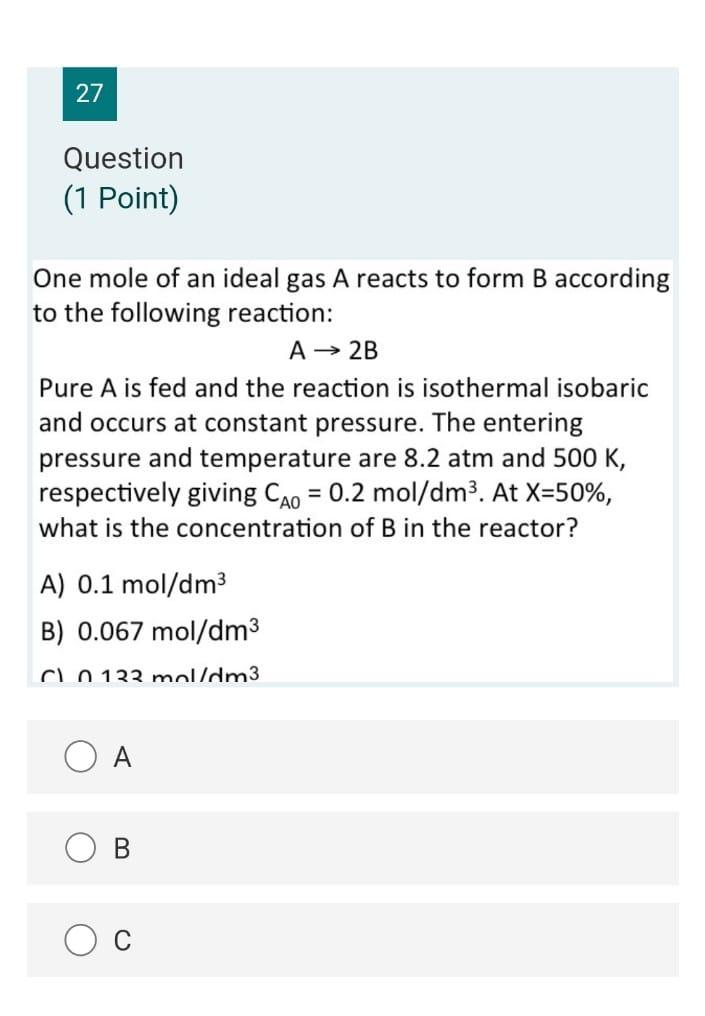
38 Question (1 Point) Part 2 A + 2 B 2 C+D What is the liquid phase concentration of B as a function of initial conversion of A if the concentration of B is 3 times that of A, (i.e., = 3 Cao) of the problem? A) CB = 2CA0(1 X) B) CB = CB0(1-2x) C) CB = 2CA013), -X) 2 D) CR = 240(1-3X) A B C 37 Question (1 Point) A + 2 B + 2 C+D What material balance expression do you obtain when you combine the first three steps? 0.6 dx A) t= So (1-x) 0 0.6 B) 2kCaotu dx (1-x)? 0 dx C) kc ct= 0 (1-x) 0.6 dx D) akcct - So 11 - V13 B C D 36 Question (1 Point) A + 2B 2C+D What are the appropriate expressions for CA and Cg as a function of conversion of A in this liquid phase reaction with a stoichiometric feed? A) CA =CAo(1-3), CB =CAo(1-0) B) CA = CA0(1 X), Cp = 2CA0(1-X) C) CA =CAo(1-1), CB =CAo(2-x) D) CA =CAo(1-3), CB =CAo(1 2X) o 34 Question (0.5 Points) Which of the following statements is false? A) A PER is equivalent to an infinite number of CSTRs in series. B) The space time in a CSTR is equivalent to the batch time in a batch reactor. C) A PFR may be thought of in terms of an infintessimally small batch reactor that moves down a pipe. D) A CSTR is equivalent to a PFR with an infinite recycle ratio. A B C OD 32 Question (1 Point) The rate constant for an irreversible, heterogeneously catalyzed, gas-phase, 2nd order reaction A Product(s) -r = P was determined to be 0.234 from experimental data in a packed-bed reactor. Proper units for the rate constant are; A) liter/(atm-gcat-hr) B) liter/(mol-gcat-hr) C) liter2/(atm-gcat-hr) D) mol/(atm-gcat-hr) A B C 27 Question (1 Point) One mole of an ideal gas A reacts to form B according to the following reaction: A 2B Pure A is fed and the reaction is isothermal isobaric and occurs at constant pressure. The entering pressure and temperature are 8.2 atm and 500 K, respectively giving Cao = 0.2 mol/dm3. At X=50%, what is the concentration of B in the reactor? = A) 0.1 mol/dm3 B) 0.067 mol/dm3 Co 122 mol/dm3 B
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


