Question: 38. The equilibrium constant, Kp, is 2.4103 at a certain te perature for the reaction 2NO(g)N2(g)+O2(g) For which of the following sets of conditions is
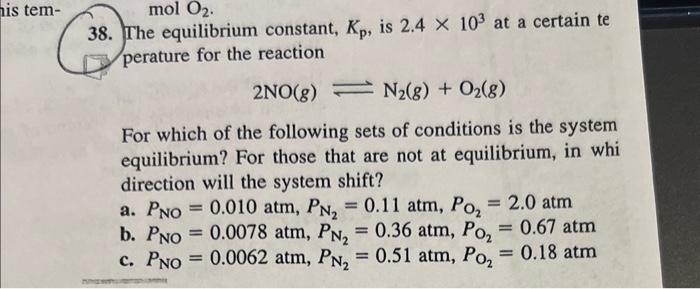
38. The equilibrium constant, Kp, is 2.4103 at a certain te perature for the reaction 2NO(g)N2(g)+O2(g) For which of the following sets of conditions is the system equilibrium? For those that are not at equilibrium, in whi direction will the system shift? a. PNO=0.010atm,PN2=0.11atm,PO2=2.0atm b. PNO=0.0078atm,PN2=0.36atm,PO2=0.67atm c. PNO=0.0062atm,PN2=0.51atm,PO2=0.18atm
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


