Question: If two krypton atoms are held together by a stabilization energy of 1.005 kJ/mol at what temperature would you expect these atoms to transition
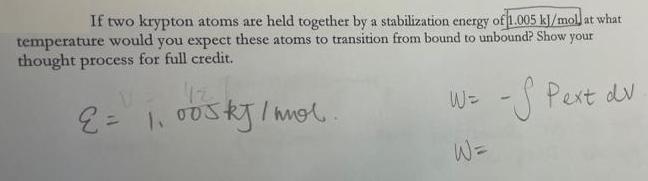
If two krypton atoms are held together by a stabilization energy of 1.005 kJ/mol at what temperature would you expect these atoms to transition from bound to unbound? Show your thought process for full credit. W= - Pext dv W=
Step by Step Solution
★★★★★
3.37 Rating (150 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

stabilization energy the interaction released on en... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


