Question: 4. A+ 2B - C + D is a liquid phase reaction carried out in a flow system reactor and is first order in both
4. A+ 2B - C + D is a liquid phase reaction carried out in a flow system reactor and is first order in both A and B with k = 0.001 dm^/(mol min) at 450 K with activation energy E= 19870 cal/mol. The feeding molar ratio between A and B is 1:2 with Fro =300 mol/min. The feeding volumetric flow rate of A and B are 50 dmmin and '100 dm'/min, respectively. (Hint: gas constant = 1.987 cal/(K-mol)
- Set up a stoichiometric table for this reaction. (10%)
- What would be the corresponding volume of CSTR to achieve 90% conversion at
600 K? (10%)
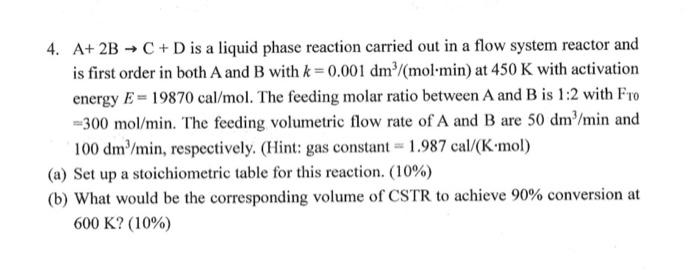
4. A+2BC+D is a liquid phase reaction carried out in a flow system reactor and is first order in both A and B with k=0.001dm3/(molmin) at 450K with activation energy E=19870cal/mol. The feeding molar ratio between A and B is 1:2 with FT =300mol/min. The feeding volumetric flow rate of A and B are 50dm3/min and 100dm3/min, respectively. (Hint: gas constant =1.987cal/(Kmol) (a) Set up a stoichiometric table for this reaction. (10\%) (b) What would be the corresponding volume of CSTR to achieve 90% conversion at 600K?(10%)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


