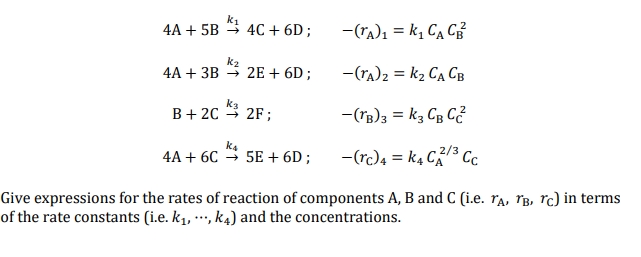
Question: 4 A + 5 B k 1 4 C + 6 D ; , - ( r A ) 1 = k 1 C A
;
;
;
;
Give expressions for the rates of reaction of components A B and ie in terms
of the rate constants iecdots, and the concentrations.Problem Consider the following set of multiple reactions: ABkCD;rAkCACBABkED;rAkCACB BCkF;rBkCBCCACkED;rCkCACC Give expressions for the rates of reaction of components AB and C ie rArBrC in terms of the rate constants ie kcdots,k and the concentrations.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


