Question: 4) a) A reaction chamber is divided into two sections by a partition on which a valve is inserted. One section contains pure gas A
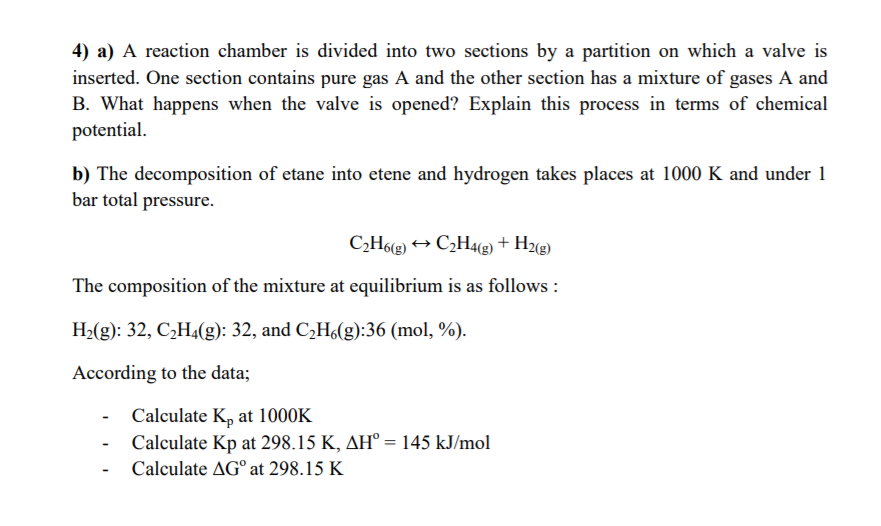
4) a) A reaction chamber is divided into two sections by a partition on which a valve is inserted. One section contains pure gas A and the other section has a mixture of gases A and B. What happens when the valve is opened? Explain this process in terms of chemical potential. b) The decomposition of etane into etene and hydrogen takes places at 1000 K and under 1 bar total pressure. C2H6(g) + C2H4(g) + H2(g) The composition of the mixture at equilibrium is as follows: H2(g): 32, C2H4(g): 32, and C2H6(g):36 (mol, %). According to the data; Calculate K, at 1000K Calculate Kp at 298.15 K, AH = 145 kJ/mol Calculate AG at 298.15 K
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


