Question: 4) A chemist uses a reaction chamber to study the reaction of C3H8 (propane) and C5H10 (pentene). with OH radicals. The reaction rate constants for
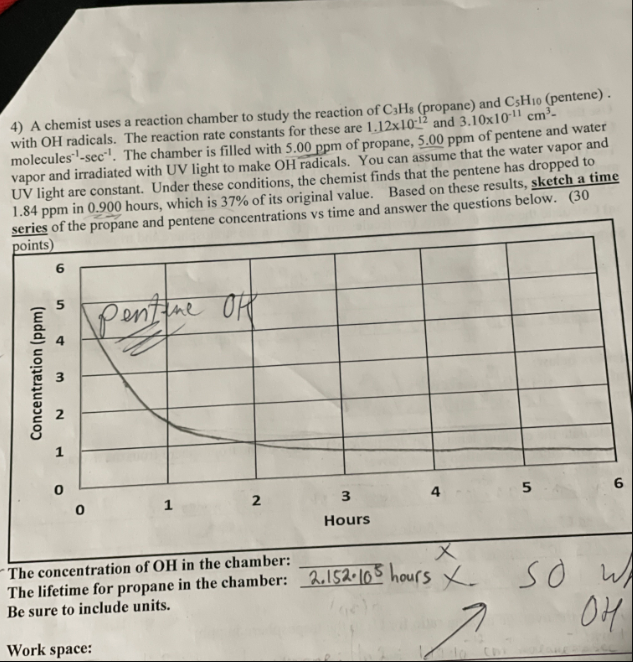
4) A chemist uses a reaction chamber to study the reaction of C3H8 (propane) and C5H10 (pentene). with OH radicals. The reaction rate constants for these are 1.121012 and 3.101011cm3 molecules 1sec1. The chamber is filled with 5.00ppm of propane, 5.00ppm of pentene and water vapor and irradiated with UV light to make OH radicals. You can assume that the water vapor and UV light are constant. Under these conditions, the chemist finds that the pentene has dropped to 1.84ppm in 0.900 hours, which is 37% of its original value. Based on these results, sketch a time The lifetime for propane in Be sure to include units. Work space
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


